Advances in Animal and Veterinary Sciences
Research Article
Blood Biochemical Parameters and Immunohistochemistry of Superoxide Dismutase in Rats with Exercise and Given Tempe Extract
I Nyoman Suarsana1*, Iwan Harjono Utama1, I Made Kardena2, I Nyoman Mantik Astawa3
1Biochemical Laboratory, Faculty of Veterinary Medicine, Udayana University, Bali, Indonesia; 2Pathology Laboratory, Faculty of Veterinary Medicine, Udayana University, Bali, Indonesia; 3Immunology Laboratory Faculty of Veterinary Medicine, Udayana University, Bali, Indonesia.
Abstract | Muscle activity in intensive physical exercise increases the production of oxygen radical species (ROS) and causes oxidative stress, inflammation, changes in hematological values, decreases intracellular antioxidants, and lipid peroxidation. This study aims were to determine the effect of tempe extract supplementation on the hematological profiles, interleukin-10, superoxide dismutase (SOD), and malondialdehyde (MDA) activities in excessive physical exercise rats . Fifteen male Sprague Dawley rats were randomly divided into 3 treated groups. P0 treatment (normal control rat), P1 treatment (swimming rat), and P2 treatment (rats were given tempe extract 150 mg/kg bw and swimming). Excessive-training rats were held for 1 hour every day for 4 weeks. At the end of the study blood were taken for the analysis of hematological values, interleukin-10 (IL-10), levels of malondialdehyde (MDA), SOD activity, and liver SOD expression by using immunohistochemistry. The results showed that rats that had excessive physical exercise increased the blood hematology profiles i.e.: erythrocytes, leukocytes, Hgb, blood hematocrit, increased IL-10 levels, MDA levels, while SOD activity decreased. Supplementation of 150 mg/kg bw tempe extract in excessive physical exercise rats could inhibit the formation of MDA and was able to modulate SOD. Tempe extract supplementation given during excessive physical exercise in the rats did not significantly change the hematological profiles and IL-10, and was not significantly different (P>0.05) compared to the normal control group. It can be concluded that excessive exercise in rats increases the levels of IL-10, MDA and decreases SOD activity. Giving tempe extract during excessive exercise maintains the leukocyte profile and IL-10 levels close to their normal values and is able to inhibit the formation of MDA and increases SOD activity.
Keywords | Biochemical, Exercise, Hematology, Interleukin, Tempe
Received | May 14, 2021; Accepted | June 12, 2021; Published | July 28, 2021
*Correspondence | I Nyoman Suarsana, Biochemical Laboratory, Faculty of Veterinary Medicine, Udayana University, Bali, Indonesia; Email: suarsana65@unud.ac.id
Citation | Suarsana IN, Utama IH, Kardena IM, Astawa INM (2021). Blood biochemical parameters and immunohistochemistry of superoxide dismutase in rats with exercise and given tempe extract. Adv. Anim. Vet. Sci. 9(9): 1324-1330.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1324.1330
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Suarsana et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Physical exercise is considered as a beneficial factor for health. However, excessive physical exercise is also known to induce oxidative stress, inflammation, and muscle fatigue (Simioni et al., 2018). Physical exercise also triggers a systemic inflammation response by releasing cytokines (Smith et al., 2013), responses to the immune system (lymphocytes, white blood cells) and hematological values (Gjevestad et al., 2015).
The beneficial effects of excessive physical exercise cause the release of myokines (Allen et al., 2015). These molecules can exert and have an auto endocrine effect and include cytokines, interleukin-6, interleukin-10, and other peptides that are produced and released by muscle fibers and have a role in protection against inflammation (Rodriguez et al., 2014).
The intensity of excessive physical exercise increases oxygen consumption, especially in the skeletal and cardiac muscles. According to Sen (2013), the use of muscle oxygen during strenuous exercise can increase 100-200 times than at rest. Increased oxygen consumption will increase the activation of certain metabolic pathways during physical exercise and produce reactive oxygen species such as: superoxide (O2-), H2O2, and hydroxyl radicals (OH-). The excessive increase in ROS during heavy physical exercise can damage cell membranes, muscle performance, macromolecules, and decrease cellular function (Simioni et al., 2018).
Lipid peroxidation is oxidative lipid degradation which results in the final product of malondialdehyde (MDA) and 4-hydroxynonenal (HNE) (Anthonymuthu et al., 2017). This molecule is known as a major bioactive marker of lipid peroxidation caused by biological activity with ROS (Kim et al., 2015). Organisms are equipped with antioxidant defense systems that protect cells from the toxic effects of free radicals. The body has enzymatic antioxidant defenses namely, the enzyme superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) and non-enzymatic antioxidants (Kawamura and Muraoka, 2018). Antioxidants will change free radicals into stable compounds, so it does not damage the cell components.
In addition to endogenous antioxidant systems, some natural compounds that are usually supplied in food can act as exogenous antioxidants and important ergogenic factors in physical exercise, one of which is tempe. Tempe is a traditional Indonesian product made from soy fermentation and is a functional food source of protein, amino acids, antioxidants, and nutraceuticals (Lakshmy et al., 2015; Wolkers et al., 2018). Exogenous antioxidant function is to prevent or reduce oxidative stress, muscle fatigue, yet improve physical activity performance (Wu et al., 2018).
The extent to which physical exercise can produce beneficial or dangerous free radicals depends on the duration of the exercise, its intensity, condition and nutritional status of the individual. When free radical production is excessive and accumulated in the body, they will be very dangerous because free radicals can damage cells and lead to destruct their function. It is therefore, necessary to make exogenous antioxidant supplementation efforts to prevent or reduce the effects of oxidative stress caused by excessive physical exercise. This study aims to determine the effect of tempe extract supplementation on the hematological profile, interleukin-10, superoxide dismutase (SOD) activity, malondialdehyde (MDA) levels and liver SOD expression by immunohistochemistry in rats with swimming exercise.
MATERIALS AND METHODS
This research was conducted by following the rules of ethics for animal welfare and animal rights, with the approval (No:1242A/UN14.2.9/PD/2019) for experimental animals of the Ethics Committee of the Faculty of Veterinary Medicine, Udayana University.
Experimental animals
Fifteen males of 10 weeks old Sprague Dawley rats (190±5 g) were divided into 3 treatments (5 for each treatment) randomly placed in individual cages. They were placed in a room with a temperature of 25-29oC and a relative humidity of 50-60%. During the study, the animals were given commercial pellet feed, drinking water adlibitum and the cages were cleaned every day. Rats were divided into 3 treatments: (1) P0 treatment: normal control rats were fed commercial pellets and not given tempe extracts or not forced to swim; (2) P1 treatment: rats were given commercial pellet feed and forced to swim for 1 hour / day; and (3) P2 treatment: rats were given commercial pellet feed, forced to swim 1 hour/day, and tempe extract 150 mg/kg body weight (bw)/day. In the P2 treatment group, swimming and tempe extract were given for 4 weeks. Tempe extract and its phytochemical contents were prepared based on previous studies conducted by Suarsana et al. (2020). The extract is given orally using a gastric sonde.
Swimming Exercise
Swimming in the rats was performed in the morning starting at 08.00-09.00. Thirty minutes after being treated with tempe extract. The rats were placed in a box (50x50x50 cm) filled with a water at a temperature of 25-26oC with its depth of 40 cm. Rats were observed for swimming and confirmed to be moving for an hour. After the exercises, the rats were returned to their original cages.
Sample collection
At the end of the treatment, all rats were anesthetized using ketamine-HCl. The blood was drawn from the orbital vein using a capillary tube and collected into a tube containing EDTA and homogenized. Each sample blood was divided, a apart to be analyzed for the hematological profile values. Another part of the blood was centrifuged at 10.000 rpm for 15 minutes to get the plasma. Plasma was analyzed to determine interleukin-10 activity. Next, the rats were euthanized and dissected to collect their livers. Each sample of the liver tissue was partly accommodated to be used for analyzing the liver SOD and MDA activity, while in the other part, the liver was placed in a tube containing 10% neutral buffered formalin for immunohistochemistry evaluation.
Table 1: The erythrocyte profile of rats supplementation tempe extract and exercise.
| Treatments |
RBC (x103/µl) |
PCV (%) |
Hgb (g/dl) |
MCV (fl) |
MCH (pg) |
MCHC (g/dl) |
| P0 | 6.97±0.52a* | 37.62±2.30a | 12.76±0.92a | 53.70±0.74a | 18.30±0.16a | 34.06±0.48a |
| P1 | 7.76±0.57b | 40.34±2.13ab | 13.82±0.45ab | 54.32±1.88a | 18.40±0.23a | 34.74±0.13a |
| P2 | 7.8±0.55b | 40.74±2.48b | 14.16±1.04b | 53.66±1.83a | 18.32±0.19a |
34.60±0.93a |
* Different letters at the same column indicate significant differences between groups for the same. P0 (normal control); P1 (swimming exercise); P2 (swimming exercise and given tempe extract of 150 mg/kg body weight / day). RBC (Red blood cell count); PCV (Packed cell volume); Hgb (hemoglobin concentration); MCV (Mean corpuscular volume); MCH (Mean corpuscular hemoglobin) (pg); MCHC (Mean corpuscular hemoglobin concentration) (g/dl)
Table 2: The leucocyte profile of rats supplementation tempe extract and exercise.
| Treatments |
Leukocyte (x103/µl) |
Leucocyte differential (x103/µl) |
|||
| Lymphocyte | Neutrophil | Monocyte | Eosinophil | ||
|
P0 |
7.24±0.63a* | 4.25±0.76a | 2.29±0.25a | 0.60±0,09a | 0.11±0,07a |
| P1 | 9.18±0.69b | 6.09±0.69b |
2.47±0.45a |
0.52±0,08a | 0.11±0,08a |
| P2 | 8.23±0.66ab | 5.26±0.61b | 2.39±0.35a | 0.49±0,08a |
0.10±0,06a |
* Different letters at the same column indicate significant differences between groups for the same. P0 (normal control); P1 (swimming exercise); P2 (swimming exercise and given tempe extract of 150 mg/kg body weight/day)
Hematological analysis
Hematologic analysis was performed using an automated hematology analyzer (Barros et al., 2018) using Sysmex XS-8001 multispecies hematology analyzer. Hematological analysis of the blood included red blood cell count (RBC), hemoglobin concentration (Hgb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), white blood cell (WBC), and leukocyte differential.
Interleukin-10, Superoxide dismutase (SOD), and malondialdehyde (MDA) activity assay
The activity of interleukin-10 (IL-10), SOD, and MDA was determined using a commercial diagnostic kit. Interleukin activity was determined using an rat IL-10 (Interleukin 10) ELISA Kit (Cat.No.:E-EL-R0016, Elabscience). SOD activity was determined using the SOD Assay Kit Catalog No: E-BC-K020, Elabscience), and MDA was determined using the MDA ELISA Kit (Cat. No: E-EL-0060, Elabscience). The analysis was carried out according to the recommended procedure in each of the kit product.
SOD analysis using immunohistochemistry
The liver tissue was fixated for 24 hours in a 10% formalin buffer solution, before it then processed by standard tissue evaluation methods using a paraffin block, cut into 0.5 nm size and attached to a glass object. The tissue was incubated with H2O2 in methanol for 15 minutes. Furthermore, it was dribbled with 10% bovine serum albumin (BSA) for 45 minutes at the temperature of 37°C. After washing, the tissue was dribbled with anti-SOD monoclonal primary antibodies and left at room temperature for an hour. Furthermore, the tissue was also dribbled with N-Histofine Simple Stain MAX PO (multi) Universal immuno-peroxidase Polymer (LOT 1708, Nichirei Biosciences Inc) for 30 minutes. The results of the antigen-antibody reaction were visualized using diamino benzidine at room temperature for 5 minutes, then countered with hematoxylin. At each treatment stage, the tissue was washed with PBS 3 times. Observation was made under a light microscope using 200 times magnification. The brown color observed in the tissue cells indicated SOD positive results.
Statistical analysis
The data obtained were analyzed in variance analysis (ANOVA) using SPSS software version 22.
RESULTS AND DISCUSSION
Hematological profiles and levels of interleukin-10
The hematological profiles of erythrocyte rats included the red blood cell count (RBC), hemoglobin concentration (Hgb), packed cell volume (PCV) or haematocrite, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCV) MCHC) is presented in Table 1. Test results for the analysis of variants of the erythrocyte profiles in the experimental animals showed significant effects (P<0.05) in the number of red blood cells (erythrocytes), Hgb, and hematocrit (PCV) (P<0.05). In general, in rats that treatead with tempe extract (P2) increased significantly higher (P<0.05) in their Hgb, hematocrit, and erythrocyte values compared to the P0 treatment. Meanwhile, the MCV, MCH, and MCHC levels were not significantly different (P>0.05) (Table 1).
Analysis of variance test results showed the values of leukocytes and lymphocytes in the excessive exercise treatment (P1) were significantly higher (P<0.05) compared to the control treatments (P0). However, the values of leukocytes and lymphocytes in the tempe extract group (P2) were lower compared to the swimming treatment (P1) although it was not significantly different (P>0.05). In addition, the values of neutrophils, monocytes and eosinophils in the three treatment groups were not significantly different (P>0.05) (Table 2)
Interleukin-10 levels
The variant test results showed that the rats in the treatment group of excessive exercise activity (P1) had increase in their IL-10 levels. The value of interlukin-10 in the swimming group had 11.14 pg/ml which was higher than the control group (P0), which had 9.95 pg/ml eventhough they were not significantly different (P> 0.05). In the rats that treated with tempe extract (P2 treatment) had non significantly lower value (P> 0.05) of the interleukin-10 at 10.62 pg/ml compared to the P1 group (Figure 1).

Figure 1: Interleukin-10 levels in the plasma rats. P0 (normal control); P1 (swimming exercise); P2 (swimming exercise and administered tempe extract of 150 mg/kg body weight/day)
Superoxide dismutase (SOD), and malondialdehyde (MDA) activity
The variant test results showed that the treatment had a significant effect (P<0.05) on SOD and MDA levels. SOD levels (78.59 U/g) were significantly lower (P<0.05) in the excessive exercise rats (P1) compared to the control treatments (P0) (110.07 U/g). In contrast, MDA levels (69.97 pmol/g) were significantly higher (P<0.05) compared to P0 (49.74 pmol/g). While no significantly different (P>0.05) observed in the average levels of SOD and MDA in the rats that had excessive physical exercise and administration of tempe extract (P3) compared to the control treatment group (P0). Results of the analysis of SOD and MDA levels in the plasma of experimental rats are presented in Table 3.
Table 3: SOD activity and MDA levels in plasma of treated rats
| Treatment |
Level of SOD (U/g) |
Level of MDA (pmol/g) |
| P0 | 110.07±12.74a | 49.74±3.83a |
| P1 | 78.59±11.1b | 69.97±4.73b |
| P2 | 97.48±12.52a | 47.79±3.97a |
*Different letters at the same column indicate significant differences between groups. P0 (normal control); P1 (swimming exercise); P2 (swimming exercise and given tempe extract of 150 mg/kg body weight/day)
Immunohistochemical SOD analysis
The results of immunohistochemical staining of the rat liver tissue treatment of SOD antioxidant enzymes showed the tissue containing SOD antioxidants in the nucleus and cytoplasm of liver cells marked with brown color (Figure 2). In the P0 group, strong brown intensity was observed indicated the SOD profile found in the nucleus and cytoplasm cells of the liver tissue. However, in the P2 treatment, the intensity of the brown color in the nucleus and cytoplasm cells was weaned. Even, the intensity of the brown color in the cells of the liver tissue of P1 treatment group was getting more faded away.
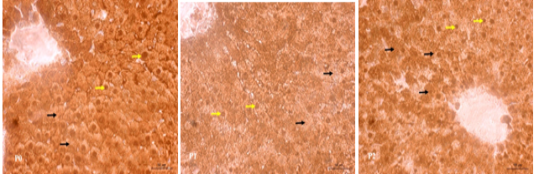
Figure 2: Immunohistochemical SOD staining in treated rat liver tissue. The SOD profile in the cell nucleus (yellow arrow) and cytoplasm (black arrow) is brown indicating the positive reaction. P0 (normal control); P1 (swimming exercise); P2 (swimming exercise and given tempe extract of 150 mg/kg body weight /day).
In this study, the range of hemoglobin values was between 12.7-14.16 g/dl, PCV or hematocrit between 37.6 and 40.74%, erythrocytes between 6.97 x 106 cells/µl and 7.80 x 106 cells/µl, and leukocytes between 7.24 x 103 cells /µl and 9.18 x 103 cells/µl are still within the normal limits of rat hematology. Some researchers reported the normal values of rat erythrocytes between 7.3 and 7.6 x103 cells /µl, PCV between 46.3 ± 0.6% (Barros et al., 2018), the number of rat leukocytes between 2.84 and 7.14x103 cells/µl (Aboderin and Oyetayo, 2006), and hemoglobin values between 10.6 and 14.2 g/dl (Ajibade et al., 2012).
The average hemoglobin value of treatment group P1 and P2 was not significantly different (P> 0.05), whereas both of the hemoglobin values were significantly higher (P<0.05) when compared to the P0 group. The high of the hemoglobin value found in the P2 group, may be contributed by the administration of tempe extract. Tempe is a good source of minerals, such as: iron, calcium, and zinc. Tempe contains iron around 3.6 mg/100g (Lakshmy et al., 2015). Iron in the form of Fe-transferrin helps the synthesis of Hgb (Kullisaar et al., 2001), because the Fe mineral is a constituent component of Hgb.
The number of erythrocytes in the groups of P1 and P2 was significantly higher (P <0.05) compared to the P0 group. Administration of tempe extract in P2 may play a role in helping the formation of red blood cells. Erythropoiesis is the process of formation of erythrocytes which includes morphological changes in producing erythrocytes. Erythropoiesis requires erythropoietin stimulation. Tempe is known to contain vitamin B12 which is important for human diets. Estimated values of vitamin B12 in fresh tempe ranging from 0.7 μg/100g to 8 μg /100g (Wolkers et al., 2018). The presence of vitamin B12 in tempe is special because it is very necessary in the formation of red blood cells. In addition, other bioactive components of tempe extract are thought to work through stimulation of increased activity of enzymes involved in the erythropoiesis process.
An increased number of leukocytes in P1 treatment was thought to be due to excessive physical exercise that could cause injury to the muscles. Acute physical exercise triggers a pro-inflammatory response and triggers an inflammatory cascade reaction (Allen et al., 2015), thereby stimulating the cellular immune response of leukocyte cells. According to Uddin et al. (2014), increased production of leukocytes (WBC) and its divergence are generally considered to be markers of stress and defense mechanisms triggered by the immune system against various inflammatory conditions. Tempe extract is able to prevent oxidative stress which has an impact on reducing injury or preventing cell damage, in which in the group of tempe extract and excessive exercise (P2), the number of leukocytes cells was lower than the treatment of excess exercise (P1).
Protein is an integral and important component of the diet, especially for athletes (Jäger et al., 2017). Protein supplementation during exercise increases muscle resistance and stamina (Morton et al., 2018). Tempe is a source of nutraceutical antioxidants as well as providing protein. Protein is needed as a component of immune cell membrane proteins, such as red and white blood cells and immunoglobulins. According to Knuiman et al. (2018), protein and amino acids in food can help to support exercise response and stamina. Immune cells need sufficient levels of amino acids for their structure and function (Forbes and Bell, 2019) and protein supplementation is beneficial for immune function and endurance during exercise (Cruzat et al., 2014).
Physical exercise causes damage to muscles in which their regeneration process involves a balance between pro cytokines and anti-inflammatory (Philippou and Maridaki, 2012). Damage to muscle organs can affect other system in the body, such as the immune system (Pedersen, 2013). Intensive exercise also triggers an inflammatory cascade. During and after acute exercise, cytokines released from muscles mediate metabolism and the inflammatory process (Allen et al., 2015). The immune system is also directly involved in cellular and molecular mechanisms in muscles to help cleanse necrotic tissue after strenuous exercise by macrophages, neutrophils, and lymphocytes (Philippou and Maridaki, 2012).
Interleukin-10 (IL-10) is an inflammatory marker that functions to regulate Th1 cytokines, increase B cell survival, proliferation, and antibody production and is able to block NF-κB activity (Gjevestad et al., 2015). Interleukin-10 is an important pleiotropic immunoregulatory cytokine mainly secreted by macrophages, but also by Th1 lymphocytes, cytotoxic T cells, B lymphocytes, monocytes and mast cells (Trifunović et al., 2015). The role of active compounds in plant extracts of Salvia officinalis on immunological and inflammatory parameters has been investigated by Khudiar and Hussein (2017). It has been explained that the active substance of Salvia officinalis extract can affect the production of interleukin and immunoglobulin G in normal and oxidative stress rats and it is suspected that the active compound of this extract acts on augmentation of humoral and cellular responses. Tempe has been known to contain phytochemical compounds as reported by Suarsana et al. (2020).
Superoxide dismutase (SOD) is an important molecule in the body’s defense against O2. SOD can catalyze radical O2- to oxygen or hydrogen peroxide (H2O2) and subsequently to H2O. Tempe extract supplementation resulted in higher SOD activity compared to the non-supplemented group (Table 3). This study shows the positive effect of supplementing tempe extract in rats with excessive exercise on endogenous antioxidant defenses in liver tissue (Figure 1).
MDA levels decreased in the treatment of tempe extract and excessive physical exercise (P2), this shows the role of tempe active compounds in neutralizing free radicals so as to prevent lipid peroxidation to produce MDA products. Bioactive compounds found in tempe are able to protect cells due to the effects of free radicals. Tempe contains isoflavone compounds and has high antioxidant activity to neutralize free radicals into non-radical molecules (Kuligowski, et al., 2017).
MDA products are biomarkers of tissue damage to measure oxidative stress levels. MDA is formed during the decomposition of polyunsaturated fatty acids caused by free radical attack (Li et al., 2017). Significant increase in MDA levels was measured in the excess exercise treatment (P1). Increased levels of MDA in the group can be caused by ROS caused by excessive exercise activity compared with the P0 and P2 treatments which showed MDA levels that were much lower than the P1 treatment.
Tempe extract research that was assembled with the appearance of exercise performance is still very little. When associated with a class of active compounds, tempe is rich in isoflavone compounds. Tempe containing isoflavone compound 5.307 mg/g, including daidzein isoflavone 44.45 μg/g d.m, glisitein 27.53 μg/g d.m, and genistein 46.11 μg/g d.m (Kuligowski et al., 2017). Previous studies have reported that polyphenols, such as quercetin, flavonoids, positively affect exercise performance (Nieman et al., 2010). In addition, recent studies in rats and humans have shown that supplementation of hesperedin extract (hesperidin aglycone) for five weeks in rats and four weeks in athletics has positively improved exercise performance (Estruel et al., 2019). Excessive physical exercise increases the production of ROS and lipid peroxidation products such as MDA in various organs, which cause oxidative stress in the organism (Anthonymuthu et al., 2017). Giving tempe extract to rats with excessive exercise, can prevent lipid peroxidation so that the MDA product decreases.
Active compounds with potential antioxidants found in food and medicinal plants as well as endogenous antioxidants, such as: SOD, Catalase, and Glutathione Peroxidase are the potent scavengers for free radicals (Nowfel et al., 2017). Antioxidant SOD catalyzes superoxide anion (O2-) to hydrogen peroxide (H2O2) which is then catalyzed by enzymes catalase and glutathione peroxidase (GPx) quickly catalyzed into oxygen (O2) and water (H2O) (Simioni et al., 2018). The high free radicals during excessive physical exercise lead to the use of high endogenous antioxidants such as SOD, so the expression of SOD in liver cells is greatly decreased as it can be seen from the reduced intensity of the brown color in the cells of the rat liver tissue of the swimming group (P1) (Figure 2) in this study.
CONCLUSION
The results of the study concluded that excessive physical exercise in rats may increase the profile of leukocytes, anti-inflammatory response of IL-10, MDA levels, and decrease SOD activity. Supplementation of tempe extract in rats with excessive physical exercise may increase the value of the erythrocyte profile, maintain the leukocyte profile and IL-10 levels close to the normal range value, prevent oxidative stress by inhibiting the formation of MDA, and maintain the levels and expression of endogenous antioxidant SOD in the liver tissue.
ACKNOWLEDGMENTS
We thank to the Directorate of Research and Community Research, Directorate General of Research and Development Strengthening the Ministry of Research, Technology and Higher Education of the Republic of Indonesia through Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) for the grant with the contract number: 492.57/UN14.4.A/LT/2019, March 11, 2019.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHORS’ CONTRIBUTION
I Nyoman Suarsana, made a research design and conducted data analysis; Iwan Harjono Utama analyzed and interpreted the data, I Made Kardena conducted experiments and analyzed immunohistochemical, and I Nyoman Mantik Astawa analyzed immunological parameters. All
of the authors have contributed to the contents, drafted and approved the final manuscript.
REFERENCES





