Advances in Animal and Veterinary Sciences
Research Article
Effects of Saccharomyces cerevisiae and Aspergillus oryzae Supplementation in Swamp Roughage Haylage-Based Rations on in vitro Rumen Fermentation Characteristics and Methane Gas Emission
Riswandi1*, Asep Indra M Ali1. Afnur Imsya1, Sofia Sandi1, Basuni Hamzah2, Agus Supriadi3
1Department of Animal Science, 2Department of Agricultural Product Technology, 3Department of Fishery Product Technology, Faculty of Agriculture, Universitas Sriwijaya. Jl. Raya Palembang-Prabumulih KM. 32, Indralaya, South Sumatra, Indonesia, 30662.
Abstract | Swamp roughage haylage is produced by anaerobic fermentation of swamp roughage to provide feed for future use. Feeding haylage as a single feed source in ruminant animals cannot meet the requirements for rumen microbes and host animals to develop optimally. Supplementation of probiotic is then needed. This study aimed to assess the effects of Saccharomyces cerevisiae and Aspergillus oryzae supplementation in swamp roughage haylage-based rations on rumen fermentation characteristics and methane gas emission. Measurements were taken on dry matter digestibility (DMD), organic matter digestibility (OMD), rumen pH, N ammonia (N-NH3) content, total volatile fatty acid (TVFA) concentration, partial VFA concentration, acetate-propionate (C2/C3) ratio, methane gas concentration, and total bacterial count. A completely randomized design with 4 treatments and 4 replicates was used. Treatments consisted of rations containing 70% haylage + 30% concentrate + 0 g probiotic (control), control + 0.05 % Saccharomyces cerevisiae (SC), control + 0.05% Aspergillus oryzae (AO), and control + 0.025 % Saccharomyces cerevisiae (SC) + 0.025 % Aspergillus oryzae (AO). Data were subjected to an analysis of variance and a Duncan multiple range test. Results showed that the probiotics supplementation significantly (P<0.05) increased DMD, OMD, rumen pH, TVFA concentration, partial VFA concentration, and total bacterial count but reduced N-NH3 content, C2/C3 ratio, and methane gas production. It was concluded that combination 0.025 % Saccharomyces cerevisiae and 0.025 % Aspergillus oryzae in swamp roughage haylage-based rations gave the best increase in the ration digestibility, total rumen bacterial count, rumen fermentation characteristics, and reduced methane production.
Keywords | In vitro, Methane gas, Probiotic, Rumen fermentation characteristics, Swamp roughage haylage
Received | April 08, 2021; Accepted | April 30, 2021; Published | July 01, 2021
*Correspondence | Riswandi, Department of Animal Science, Universitas Sriwijaya. Jl. Raya Palembang-Prabumulih KM. 32, Indralaya, South Sumatra, Indonesia, 30662; Email: riswandi_dya@yahoo.com
Citation | Riswandi Riswandi, Ali AIM, Imsya A (2021). Effects of saccharomyces cerevisiae and aspergillus oryzae supplementation in swamp roughage haylage-based rations on in vitro rumen fermentation characteristics and methane gas emission. Adv. Anim. Vet. Sci. 9(8): 1143-1149.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1143.1149
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Riswandi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The productivity of farm ruminant animals in tropical regions is mainly influenced by the feed quality and quantity that seasonally fluctuate. Feeding of the animals for optimal production should contain balanced nutrients to meet the requirements of rumen microbes and host animals (McDonald et al., 2010; Lazzarini et al., 2016).
One of the strategies in the ruminant management in lowland areas of South Sumatra is the feeding of potential aquatic forage of swamp areas. Swamp rice grass or Bento Rayap grass (Leersia hexandra) and water mimosa or kemon air (Neptunia oleracea Lour) are two examples of grass and legume growing in swamp areas. The seasonal roughage availability in the region results in an oversupply in rainy season and an undersupply in dry season. The feeding of low quality forage (Ali et al., 2013) could reduce nutrients digestibility and increase methane production since the high fiber fractions content (Philippe and Nicks, 2015). Preserving swamp roughages by haylage in the rainy season is one of promising solution to overcome the scarcity of feed supply in the dry season. The anaerobic process in a mixture of grass and legume haylage could preserve nutrients of the roughage. (McDonald et al., 2010; Schroeder, 2013).
Utilization local probiotics serve an alternative way to fulfill the nutrient requirements of ruminant animals particularly in a feed shortage period. However, studies on Saccharomyces cerevisiae (SC) and Aspergillus oryzae (AO) supplementation in swamp roughage haylage-based rations is limited. The SC and AO supplementation in swamp roughage haylage-based rations can improve nutrients availability for rumen microbes and host animals. The main reason of probiotic supplementation in ruminant animals is the capability of probiotics to modify rumen ecosystem, microbe population, feed digestion, and rumen fermentation processes and so reduce methane gas production (Seo et al., 2010; Khan et al., 2016). Probiotic supplementation of SC has been shown to increase digestibility rate of fiber fractions, crude protein degradation, rumen fermentability, microbe efficiency, and ruminant animal performance (Ali et al., 2015; Riswandi et al., 2020). This study was aimed at assessing the effects of SC and AO supplementation in swamp roughage haylage-based rations on rumen fermentation characteristics and methane production.
MATERIALS AND METHODS
The study was conducted at Animal Nutrition and Feed Laboratory, Faculty of Agriculture, Universitas Sriwijaya.
Haylage Production
Prior to haylage production, swamp roughages consisting of bento rayape grass and kemon air legume were chopped into 3 cm cuts and wilted for 24 hours to reduce their water content up to 40% (Jaelani et al., 2014). These wilted roughages were mixed with the ratio of 70% bento grass and 30% kemon air legume (as fed). Molasses was then added into the mixture by 5% as suggested by Riswandi (2014). The mixed roughages were moved to plastic bags silo and then compacted to ensure that anaerobic condition in the plastic bags was achieved. The bags were placed in a room at 26-28oC and incubated for 21 days.
Sample Preparation and Experimental Design
In the end of the incubation period, the haylage was taken out from the bags and dried in an oven at 60oC for 24 hours and then finely ground (1 mm). In vitro substrates consisted of the haylage (70%) and a concentrate mixture (30%) (Table 1). Nutrient contents of sample feed was determined by using an AOAC method (2005) in a proximate analysis. The amount of SC and AO probiotic supplementation was in accordance to each treatment. Nutrient composition of feeds and the ration are shown in Tables 2 and Table 3, respectively.
Table 1: Concentrate composition used in the rations
| Feedstuff | Amount (kg) As Fed based |
| Rice bran | 80 |
| Ground corn | 8 |
| Tofu by-product | 10 |
| Ultra-mineral | 0,5 |
| Salt | 0,75 |
| Urea | 0,75 |
| Total | 100 |
Table 2: Nutrient composition of haylage and concentrate (% dry matter)
| Nutrient | Haylage | Concentrate |
| Dry matter | 32.05 | 76.26 |
| Organic matter | 93.14 | 89.79 |
| Crude protein | 15.48 | 17.82 |
| Crude fiber | 22.37 | 17.78 |
| Ether extract | 3.82 | 7.88 |
| Nitrogen Free Extract | 56.13 | 55.28 |
| TDN | 66.41 | 73.54 |
| Neutral detergent fiber | 45.91 | 31.62 |
| Acid detergent fiber | 32.45 | 20.59 |
| Hemicellulose | 13.43 | 11.03 |
| Cellulose | 24,71 | 15.58 |
| Lignin | 5,79 |
3.26 |
The design used was a completely randomized design with 4 treatments and 4 replications. Treatments consisted of rations containing 70% haylage + 30% concentrate + 0 g probiotic (control), control + 0.05 % Saccharomyces cerevisiae (SC), control + 0.05% Aspergillus oryzae (AO), and control + 0.025 % Saccharomyces cerevisiae (SC) + 0.025 % Aspergillus oryzae (AO).
In Vitro Fermentation and Chemical Analyzes
The in vitro study was conducted based on the method of Theodorou dan Brooks (1990). Substrates (1 g) were put into each incubation bottle which already contained incubation medium of 90 ml buffer (pH 7.9) and 10 ml buffalo rumen fluid. Rumen fluid was taken from a 2.5-year old male swamp buffalo prior to feeding time in the morning. The buffalo was fed swamp grass (bento rayape grass) and concentrate (70:30). The rumen fluid was taken with a stomach tube and a vacuum pump. The collected rumen fluid was taken to the laboratory where it was filtered by using a 100-μm nylon sieve and added to a solution consisting of 100 ml rumen fluid and 900 ml buffer solution.
Table 3: Ingredients and nutrients composition of the experimental ration.
| Ingredients (as fed) | Ration |
| Haylage (%) | 70 |
| Concentrate (%) | 30 |
| TOTAL | 100 |
| Nutrient composition (% dry matter) | |
| Dry matter | 45.32 |
| Organic matter | 91.44 |
| Crude protein | 16.18 |
| Crude fiber | 20.99 |
| Ether extract | 5.034 |
| Free Nitrogen Extract | 55.87 |
| TDN | 68.55 |
| Neutral detergent fiber | 41.63 |
| Acid detergent fiber | 28.90 |
| Hemicellulose | 12.71 |
| Cellulose | 20.61 |
| Lignin | 5.03 |
Source: Nutrition and Animal Feed Laboratory, Faculty of Agriculture, University of Sriwijaya, 2019.
These anaerobic media were then put into incubation bottles (100 ml medium per bottle). The CO2 gas was added for an optimal aerobic condition. The filled bottles were incubated in water bath incubators (39 o C) for 48 hours. In the end of the incubation time, 2 drops of HgCl2 was added into each bottle. Then, samples were centrifuged at 4000 rpm for 10 minutes until the supernatant was separated from the residue. The supernatant was kept and analyzed for N-NH3, and volatile fatty acids (VFA) concentrations, methane production, and total bacterial count. N-NH3 concentration was determined by using modified Conway procedures (General Laboratory Procedures, 1966). VFA content was determined in a gas liquid chromatography (GLC Bruker Scion 436-GC, Bruker Daltonik GmbH, Bremen, Germany) by using a BR-Wax fame column (mmlD 0.32, 0.25 lm df) and FID detector. Supernatant preparation obtained from in vitro incubation was added with 3 mg sufosalicylic acid dihydrate and centrifuged at 12,000 rpm 7 o C for 10 minutes before it was injected into GLC column. Quantification of individual VFA was conducted by comparing it with an external standard and expressed in μmol/ml or mM. Total VFA content was measured as the sum of all individual VFA contents (AOAC, 2005). Samples of 3 mL gas resulted from fermentation were taken by using a syringe glass, stored in venoject tubes. Methane gas concentration was measured after 48-hour incubation by using a gas chromatography device (Hitachi Model 26-50). The device was equipped with an FID detector and an active carbon column of 1 m length and 3 mm diameter. The temperatures in injector, detector, and column was 190, 190, and 170oC, respectively. Gas flow rates were set at 50 ml/minute for N2 gas, 1 kg/cm2 for H2, and 1 kg/cm for air (Menke and Steingass procedures, 1988). Total bacterial count was determined by a roll tube method (Ogimoto and Imai procedures, 1988). Analysis of nutrients content was conducted on the residue of incubation which were added with 50 mL pepsin-HCl 0.20% and incubated for 48 hours. The solution was filtered by using Whatman 41 filter paper and the residue was then dried at 60oC for 48 hours.
Dry matter and organic matter digestibility was measured after 48-hour incubation. Sinterglasses were oven-dried at 105oC for 24 hours before they were weighed. Dry matter digestibility was determined by drying the incubation residue in an oven at 105oC for 24 hours. Organic matter digestibility was determined by ashing the incubation residue in a furnace at 550oC for 6 hours.
Data Analysis
Data of dry matter digestibility (DDM), organic matter digestibility (OMD), rumen pH, N ammonia (N-NH3) concentration, total volatile fatty acid (TVFA) concentration, partial VFA concentration, methane gas concentration, and total bacterial count were subjected to an analysis of variance and a Duncan multiple range test (SPSS 13.0 program).
RESULTS AND DISCUSSION
Dry Matter and Organic Matter Digestibility
Results showed that the probiotics supplementation in the rations improved (P<0.05) DMD and OMD. DMD and OMD were found to be the highest in SC+AO and those in SC, AO, and control were also different (Table 4).
The SC+AO supplementation in ration basal gave the highest DMD and OMD values. This might result from the synergy between the two kinds of probiotics used. S. Cereviseae was able to produce amylase enzyme to digest starch and AO produced cellulose and hemicellulose enzymes digesting fibers (cellulose and hemicellulose) (Alshaikh et al., 2002). In addition, SC is a growth factor for cellulolytic bacteria as it provides nutrients including vitamins, minerals, and amino acids for rumen bacterial growth (Khan et al., 2016). Increased population of cellulolytic bacteria improves cellulolytic activity resulting in improved fiber digestion. This later leads to increased DM and OM digestibility. Compared to combined probiotic supplementation, individual probiotic supplementation resulted in lower DMD and OMD. The values of DMD and OMD in SC were higher than that in AO. This might be attributed to the role of yeast (SC) in providing substrates in the form of organic acids and vitamins which promotes the growth of lactic acid bacteria (LAB) (Khan et al., 2016). By stabilizing rumen pH, increased LAB population improves rumen metabolism and cellulolytic bacteria population, reduces lactic acid accumulation in digestive tract, reduces oxygen concentration through glycolysis process, improves ration digestibility and rumen fermentability, and affects animal performance (Kumar et al., 2013; Pinloche et al., 2013).
Table 4: Effect of Saccharomyces cereviceae (SC) and Aspergillus oryzae (AO) supplementations of mixed haylage (70%) and concentrate (30%) ration on Digestibility of Dry Matter (DM) and Organic Matter (OM)
| Treatment | Digestibility (%) | |
| DM | OM | |
| Control |
51.28±1.46a |
60.83±1.46a |
| SC |
57.56±0.54c |
67.77±1.76c |
| AO |
55.97±0.47b |
64.64±0.94b |
| SC+AO |
59.89±0.45d |
69.86±0.71d |
Note: Different superscripts in the same column indicate significant differences (P<0.05
Table 5: Effect of Saccharomyces cereviceae (SC) and Aspergillus oryzae (AO) supplementations of mixed haylage (70%) and concentrate (30%) ration on Fermentation Characteristics (pH, N-NH3, and Total VFA Concentration)
| Treatment | pH | N-NH3 (mM) | Total VFA (mM) |
| Control |
6.59±0.04a |
11.95±1.57c |
67.72±3.28a |
| SC |
6.70±0.05b |
7.61±1.01ab |
104.61±5.92c |
| AO |
6.69±0.06b |
8.18±1.01b |
85.63±2.97b |
| SC+AO |
6.72±0.03b |
7.18±0.38a |
122.98±3.42d |
Note: Different superscripts in the same column indicate significant differences (P<0.05).
Characteristics of rumen fermentation
Compared to that in control group, rumen pH in the probiotic supplementation groups was significantly (P<0.05) higher. Yet, no different rumen pH was found in treatment groups (Table 5). All treatments contributed to increases in rumen TVFA (P<0.05) with the highest found in SC + AO and the lowest in control (Table 5). Compared to that in control group, rumen N-NH3 contents in the probiotic supplementation groups were lowered. The lowest rumen N-NH3 content was found in SC + AO. However, no different rumen N-NH3 content was found in SC, AO, and SC + AO (Table 5).
Rumen pH
Rumen pH was elevated as the effect of the probiotics supplementation. This was indicated by rumen pH of 6.61 to 6.75 which was still in the normal range (6.2 to 7.00) (Barber et al., 2010) so that no disturbance in rumen microbial growth was found. Furthermore, in addition to providing nutrients and important cofactors stimulating rumen microbial activity, SC probiotic also plays a role in controlling rumen environment to be more anaerobic. This condition stimulates rumen microbial growth followed by improved ammonia and lactic acid utilization to stabilize rumen pH. The inclusion of SC in rations with high energy content was found to stabilize rumen pH and avoid the occurrence of acidosis (Pantaya et al., 2010). It was also reported that balanced fermentation products (VFA and N-NH3) could maintain rumen pH (Mc Donald et al., 2010).
TVFA
The content of TVFA in this study was still within normal range for the optimal activity and growth of rumen microbes. The optimal VFA content required for rumen microbial growth was 80–160 mM (Mc Donald et al., 2010). The highest VFA production which might be caused by high ration digestibility was found in SC+AO supplementation. The increased DMD was found to improve ration fermentation to produce volatile fatty acids (VFA) (Mc Donald et al., 2010). The effects of individual probiotic supplementation on TVFA content were in line with those on ration digestibility. The composition of VFA produced in rumen was affected by a fermented substrate, microbe population, and rumen ecology (Bannink et al., 2008).
N-NH3
It was reported that probiotic supplementation lowered rumen N-NH3 content (Mohammed et al., 2018) that was in line with the result of the present study. The concentration of N-NH3 resulted from protein degradation by rumen microbes are used as a nitrogen source for microbial protein synthesis (Mc Donald et al., 2010). N-NH3 concentration was found to be the highest in control indicating less N-NH3 utilization for rumen microbial growth. The N-NH3 concentration in SC and AO was not different but there were higher than that in SC+AO. This indicated that, compared to combined probiotic (SC+AO) supplementation, individual probiotic supplementation (SC and AO) resulted in lower N-NH3 utilization by rumen microbes for microbial protein synthesis. Higher N-NH3 utilization for rumen microbial protein synthesis by rumen microbes in SC+AO was reflected in higher total rumen bacterial count (Table 4). Rumen ammonia concentration found in this study was higher than the optimal N-NH3 requirement for microbial protein synthesis. Rumen microbial growth required an optimal ammonia concentration of about 4 to 21 mM (Yuan, 2010).
Partial VFA and C2/C3 Ratio
With regard to the effects of treatments on partial VFA concentration, it was found that C2 proportion was 67.99, 58.85, 58.79, and 58.67% and C3 proportion was 20.05, 25.16, 26.24, and 26.98% in control, SC, AO, SC+AO, respectively (Table 6). The means of rumen C2/C3 ratio were reduced with the probiotic supplementations with the lowest in SC+AO and the highest in control (Table 4).
Partial VFA concentration is the result of structural and non-structural carbohydrate fermentation by rumen microorganisms. Partial VFA is also produced from microbial and feed protein fermentation (McDonald et al., 2010). In this study, probiotic supplementation resulted in C2 and C3 in a proportion closed to that presented by Arora (1995), namely about 50 - 65% C2 and 18 - 24% C3.
Table 6: Effect of Saccharomyces cereviceae (SC) and Aspergillus oryzae (AO) supplementations of mixed haylage (70%) and concentrate (30%) ration on partial VFA concentration and ratio of C2/C3 production in rumen
| Treatment | C2 (mM) | C3 (mM) | C4 (mM) | Ratio C2/C3 |
| Control |
46.04± 2.54a |
13.58± 0.99a |
8.10± 0.47a |
3.40± 0.18c |
| SC |
61.50± 4.72c |
27.45± 1.41c |
15.66± 1.47c |
2.24± 0.13ab |
| AO |
50.40± 1.72b |
21.55± 0.93b |
13.69± 0.88b |
2.34± 0.11b |
| SC+AO |
72.15± 5.28d |
33.18± 1.01d |
17.65± 1.21d |
2.18± 0.22a |
Note: Different superscripts in the same column indicate significant differences (P<0.05).
C2: Acetate , C3: Propionate, C4; Butyrate
The C2/C3 ratios in SC+AO and AO were different, while in AO and SC were not different. The low C2/C3 ratio in SC+AO was caused by the notion that probiotic supplementation stimulated starch-degrading bacteria to produce propionic acid in higher concentration than acetic acid. The SC supplementation was found to decrease C2 and increase C3 (Inal et al., 2010). Meanwhile, AO supplementation was found to reduce C2 and C4 production and improve C3 production, DMD, and VFA concentration (Sosa et al., 2011). The C2/C3 ratio is an important parameter in ruminology as low C2/C3 ratio stimulates fattening and body fat formation. Propionic acid is glycogenic and the main precursor of blood glucose formation (Vlaeminck et al., 2006). High C2/C3 ratio found in control indicated that microbial fermentation pattern was directed to acetic acid formation.
Methane gas concentration
Methane gas concentration was reduced with probiotic supplementation (P<0.05) as depicted in Figure 1. Methane gas concentration in control was significantly higher than those in the SC, AO, and SC+AO groups. However, methane gas concentration among the probiotics groups were not different. The SC, AO, and SC+AO supplementation were found to increase total bacterial counts as depicted in Figure 2.
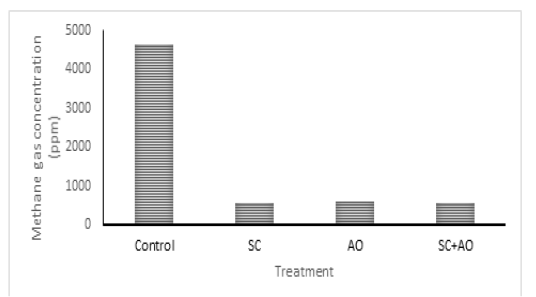
Figure 1: Effects of Saccharomyces cereviceae (SC) and Aspergillus oryzae (AO) supplementations of mixed haylage (70%) and concentrate (30%) ration on methane gas concentration
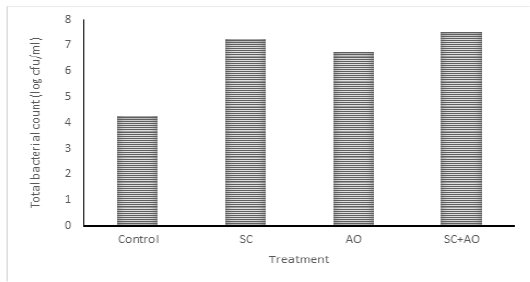
Figure 2: Effects of Saccharomyces cereviceae (SC) and Aspergillus oryzae (AO) supplementations of mixed haylage (70%) and concentrate (30%) ration on total bacterial count
The formation of methane gas produced by enteric fermentation of ruminants is determined by the type of VFA produced, the formation of propionate requires H2, while the formation of acetate and butyrate produces H2. This indicates that acetate and butyrate formation triggered the formation of H2 so that it will be used by methanogenic bacteria to be converted into CH4, while high propionate production requires H2 so that the formation of CH4 will decrease (Martin et al., 2009). The C2/C3 ratio has a closed relation with methane gas emission. Low C2/C3 ratio means low methane gas production (Mitsumori and Sun. 2008; Martin et al., 2009). It was shown in this study that C2/C3 ratio was lower in supplementation groups than in control group. Improved rumen fermentability and lower C2/C3 ratio were shown with supplementation of SC and rumen microbe isolates (Riyanti et al., 2016). Increased hydrogen supply for methanogenesis was indicated as the cause of increased C2/C3 ratio and methane gas production (Martin et al., 2009; Wallace et al., 2017). Yeast supplementation was able to stimulate acetogens to compete with methanogen in utilizing hydrogen which made methane gas emission reduced (Mwenya et al., 2004). Lowered methane gas production in rumen improved feed energy availability, which subsequently gave positive effects on animal productivity
Total Bacterial Population
The SC, AO, and SC+AO supplementation were found to increase total bacterial counts as depicted in Figure 2. Probiotic supplementation in rations increased the total rumen bacterial population as indicated by high DMD and NH3 and TVFA concentrations within the optimal limit. This condition was an advantage for the increment of microbial protein synthesis. Improved digestibility was found to be in line with enhanced microbial protein synthesis (Nurhaita et al., 2010). This occurred because of a higher supply of carbon frames from TVFA and nitrogen from N-NH3 for substrates to be used by rumen microbes for their growth (McDonald et al., 2010). Zain et al. (2011) showed that the inclusion of probiotic in ruminant rations consisting of roughage and concentrate improved bacterial population growth. This contributed to the availability of microbial protein sources supplying about 70-90% amino acid requirement of host animal (Russell et al., 2009).
CONCLUSIONS
It was concluded that combination 0.025 % Saccharomyces cerevisiae and 0.025 % Aspergillus oryzae in the swamp roughage haylage-based rations gave the best increase in the ration digestibility, total rumen bacterial count, rumen fermentation characteristics, and reduced methane emissions. This present study recommend that the probiotic combination should be tested in an in vivo study to determine the effect on the performance of ruminants.
CONFLICT OF INTEREST
We declare that in this research there is no conflict of interests.
ACKNOWLEDGEMENT
Research fund assistance from University of Sriwijaya through a featured competitive research scheme Number 0015/UN9/SK.LP2M.PT/2019 was acknowledged.
AUTHOR’S CONTRIBUTION
Riswandi: original concept, study design and conducted the experiment. Asep Indra M. Ali: involvement in conduct of the experiment. Basuni Hamzah and Agus Supriadi: responsible for statistical analysis. Sofia Sandi and Afnur Imsya: significant contribution to discussion and writing, critical revisions of the article. All authors have read and agree with the final version of the manuscript.
REFERENCES





