Advances in Animal and Veterinary Sciences
Research Article
Usage of Some Natural or Synthetic Compounds as Antioxidant and their Effects on Cryopreservation and Penetration of Ram Spermatozoa
Hala Mohamed Diab1, Osama Mohamed Ahmed1,2, Fahim H.I.1, Mahmoud Yassin Mohamed3*
1Physiology division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt; 2Physiology division, Faculty of Oral Dental Medicine, Nahda University, Beni-Suef, Egypt; 3Animal Production Research Institute (APRI), Agricultural Research Center (ARC), NC 12618, Dokki, Giza, Egypt.
Abstract | The current investigation was performed for determining the impact of natural (rosemary and ginseng aqueous extracts) and synthetic (L-carnitine and vitamin E) antioxidants on ram sperm characteristics during three sessions (preservation, equilibration, and frozen-thawing) using six mature Ossimi rams. Semen collection was performed twice a week for three weeks by an artificial vagina. In different sessions, ejaculates were pooled split into five aliquots. The natural (rosemary and ginseng aqueous extracts) and synthetic (L-carnitine and vitamin E) antioxidants were supplemented 20 mg/ml of each to the first four portions G1, G2, G3, and G4, respectively. However, the fifth part (G5) served as control without any antioxidant type. The results showed that sperm characteristics, sperm recovery rate and reduced glutathione (GSH) were higher (P < 0.05), but the concentration of lipid peroxidation (LPX) and enzymatic activities (ALT, AST, ALP, and LDH) were lower (P < 0.05) in G1, G2, G3 and G4 than G5 extenders post-equilibration and thawing conditions. Furthermore, the penetration ability into ewe cervical mucus was significantly (P < 0.05) better in the extended ram semen with natural antioxidant (G1 & G2) than with synthetic antioxidant (G3 & G4). The findings of this study indicated that extenders supplemented with rosemary or ginseng extract (as a natural antioxidant) could be alleviated sperm characteristics, penetration value and reduced oxidative stress marker corresponding to L-carnitine and vitamin E (as a synthetic antioxidant) during after the cooling and freeze-thawing processes of ram spermatozoa.
Keywords | Antioxidants, Cryopreservation, Ram spermatozoa, Penetration value.
Received | October 14, 2020; Accepted | December 12, 2020; Published | March 30, 2021
*Correspondence | Mahmoud Yassin Mohamed, Animal Production Research Institute (APRI), Agricultural Research Center (ARC), NC 12618, Dokki, Giza, Egypt; Email: dr.yassin2005@gmail.com
Citation | Diab HM, Ahmed OM, Fahim HI, Mohamed MY (2021). Usage of some natural or synthetic compounds as antioxidant and their effects on cryopreservation and penetration of ram spermatozoa. Adv. Anim. Vet. Sci. 9(5): 743-753.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.743.753
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The chilling of semen has some adverse effects on sperm properties. Modifying dilators with traditional and untraditional refrigerants can influence sperm properties (Mohamed et al., 2020). Preservation of semen alters sperm structure and biochemical properties and impedes fertilization (Mohamed et al., 2019).
The addition of antioxidants to semen diluents could be extended storage time, improve motility, decrease cellular damage, protected acrosomal membrane and raise the viability and fertilization ability of spermatozoa. Among substances with recognized antioxidants effectiveness were natural or synthetic to be added to extenders. In this context, vitamin E (synthetic non-enzymatic antioxidant) is one of the most principal lipid-soluble defensive antioxidants and has a major role as an antioxidant. Hence, Ahmadi Hamedani et al. (2016) indicated that supplementation 2mM of vitamin E as an antioxidant is suggested to improve cryopreservation techniques in ram sperm. Moreover, Motemani et al. (2017) demonstrated that concentrations of vitamin E (α-tocopherol) at 4.8 mM could be efficient for the preservation of bull spermatozoa in freezing status and conquer reactive oxygen species (ROS) accumulation. Additionally, L-carnitine (LC) is an essential amino acid and is also a necessary co-factor for the metabolism of fatty acids and plays a major role in producing metabolic energy by promoting the transfer of fatty acids to the mitochondria. The epididymal epithelium and spermatozoa produce energy from LC in epididymis fluid (Ruiz-Pesini et al., 2001). LC is among the most potent antioxidants. It works by decreasing LPX by transferring fatty acids for b-oxidation into the mitochondria to produce ATP energy (Rani et al., 2002). LC addition for buffalo semen dilators greatly enhanced their properties and safeguarded of the plasma membrane and its functional integrity in mitochondria (El-Raey et al., 2016).
Some studies have noted that many herbal medicinal plants possess great antioxidant potential. In this regard, rosemary and ginseng extracts have been studied to develop antioxidant activities in the medicinal and nutritional fields. Motlagh et al. (2014) revealed that aqueous rosemary extract could play a role as an appropriate antioxidant against cryopreservation by reducing spermatozoa motility and viability post-thawing (PT). Gad and Sayd (2015) recommended that rosemary extract has the highest amount of phenolic compounds. Thus, these compounds are a powerful antioxidant that inhibits the production of superoxide anion as well as LPX and free radical scavenging (de Oliveira et al., 2017). Also, ginseng root has been isolated in many groups of compounds such as triterpene saponins; essential oil-containing sesquiterpenes and polyacetylenes; peptidoglycans; polysaccharides; compounds containing nitrogen; and numerous ubiquitous substances like fatty acids, phenols, and carbohydrates (Tang and Eisenbrand, 1992). Gray et al. (2016) reported that ginseng-supplemented semen reduced the percentage of the motile cells. Ginseng shields against harm to chromatin and thus can be of benefit to reproductive function. Extender containing ginseng extract was appropriate for improving ram’s sperm characteristics (Safavi et al., 2014).
Much work has been done by adding natural and synthetic antioxidants to ram semen extenders. Still, information on their use in the native breed as Ossimi rams’ spermatozoa preservation and fecundity are not supported. Therefore, the current research was performed to investigate the comparative efficacy between adding natural antioxidant (rosemary and ginseng aqueous extracts) and synthetic antioxidant (vitamins E and L-carnitine) in the diluent to evaluate ram sperm quality (pre or post- frozen) and penetration score.
Material and Methods
Experimental design
Six mature Ossimi rams aged 2.55±0.89 years and an average of 62.0±1.22 kg body weight of proven fertility were used in this study. Semen samples were pooled immediately after collection to remove individual differences and plunged into a water bath held at 37 °C before evaluation. These samples were examined for the progressive motility, viability, abnormality of spermatozoa, sperm concentration and integrity acrosome. Semen samples displayed greater than 80% motility, viability and spermatozoa normality and integrity acrosome or sperm concentration 2.9×109 sperm/mL were selected for this experiment.
After primary observation, semen samples were split into five aliquots and different types of antioxidants diluted at a 1:4 (semen: diluents). The 1st part (G1), 2nd part (G2), 3rd part (G3) and 4th part (G4) were added with 20 mg/ml of each antioxidant types as rosemary aqueous extract, ginseng aqueous extract, synthetic L-carnitine (LC, Sigma-Aldrich C0158, Molecular Probes), and synthetic vitamin E (VE, dl alpha-tocopheryl is a mixture of eight stereoisomers, only one of which is the same as natural VE, Sigma CO.), respectively. However, the 5th part (G5) served as control (free of antioxidant type) (Figure 1). In general, ingredients of Tris semen extender and antioxidant levels are defined in Table (1).
Figure 1: Expermintal desien and grouping
Evaluation of microscopic sperm parameters
Evaluation of spermatozoa characteristics (progressive motility, sperm concentration, viability, abnormality) were described by Salisbury et al. (1978). Acrosomal integrity was determined, according to Didion et al. (1989). Also, sperm penetration into cervical mucus test was determined according to the score reported by Hanson et al. (1982). Diluent samples were centrifuged at 3500 rpm for 15 minutes, and the supernatant was expelled and utilized for the enzymatic test. Aspartate transaminase (AST), alanine transaminase (ALT), lactic dehydrogenase (LDH) and
Table 1: Ingredients of Tris semen extenders and antioxidant levels
| Extender ingredients | Semen extender types | ||||
| G1 | G2 | G3 | G4 | G5 | |
|
Tris (g)1 |
2.442 | 2.442 | 2.442 | 2.442 | 2.442 |
| Sodium citrate (g) | 0.145 | 0.145 | 0.145 | 0.145 | 0.145 |
| Citric acid (g) | 1.340 | 1.340 | 1.340 | 1.340 | 1.340 |
| Fructose (g) | 0.750 | 0.750 | 0.750 | 0.750 | 0.750 |
|
Antibiotic (ml)2 |
1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Egg yolk (ml) | 15.000 | 15.000 | 15.000 | 15.000 | 15.000 |
| Rosemary extract (mg) | 20 | - | - | - | - |
| Ginseng extract (mg) | - | 20 | - | - | - |
| L-carnitine (mg) | - | - | 20 | - | - |
| Vitamin E (mg) | - | - | - | 20 | - |
| Distilled water up to (ml) | 100 | 100 | 100 | 100 |
100 |
1Tris: Hydroxymethel amino methane. 2Antibiotics: Each ml of antibiotic (Streptopencid) is contained 30.000IU penicillin-G, 10.000IU sodium penicillin-G, and 50.000 micrograms streptomycin sulphate. G1& G2: Containing natural antioxidant genesis of rosemary and ginseng extracts, G3 & G4: Containing synthetic L-carnitine and vitamin E and G5: Free from any type of antibiotics and served as control.
Rosemary and ginseng extracts was extracted by 5 g of fresh leaves was added to 100 ml of boiling distilled water maintained for 10 min. Once the extract had cooled up to 25 °C and then filtered to remove the leaves before use, according to Daghigh-Kia et al. (2014).
alkaline phosphatase (ALP) activities were estimated according to Graham and Pace (1967).
Determination of oxidative and antioxidant status
Lipid peroxidation (LPX) concentration was measured. The level of LPX was estimated according to Jain et al. (1989) by measuring the level of malondialdehyde acid (MDA) using commercial kit LPX-586 (Oxis Research, Burlingame, CA, US) with sensitivity at 0.5µM and 0.5 to 4.0 µM as range curve. The concentration of reduced glutathione (GSH) measured, according to Ellman (1959) using a commercial kit (Bio-diagnostic company Egypt). The absorbance was measured at 405 nm.
Preservation of unfrozen-semen
Inclusive of either incubation time at 37 °C for up to 4 hours or storage time at 5 °C for up to 4 days in G1, G2, G3, G4 and G5 extenders. Ten replication samples/extender types / procedures were used. Percentage of sperm motility (%), live spermatozoa (%), abnormal spermatozoa (%), intact acrosome of spermatozoa (%) and penetration ability were checked during incubation at 37 °C for 0, 1, 2 and 4 hours or storage at 5 °C for 0, 1, 2 and 4 days. Also, enzymatic activity (ALT, AST, ALP, & LDH), oxidative stress marker (LPX) and antioxidant enzyme (GSH) were determined post-equilibration.
Cryopreservation of frozen-semen
Comprehensive freezing steps consisted of equilibration period at 5 °C for up to 120 -180 minutes, freezing in liquid nitrogen at -196 °C and thawing at 37 °C for up to 60 seconds with G1, G2, G3, G4 and G5 extenders. All previous parameters were evaluated during post- equilibration and post-thawing.
Semen samples were filled in 0.50 ml straws and sealed with polyvinyl chloride (PVC) powder and dried. After straws passed equilibration period, straws were frozen horizontally on metal rack presented in a foam box, then liquid nitrogen (LN2) vapour just 6 cm above the surface of LN2 for 10 minutes before they were plunged into LN2. The frozen straws were then transferred to a liquid nitrogen container. The straws were thawed at 37 ºC for 60 seconds after LN2 storage, and then previous characteristics were examined after 24 hours.
The freezability of spermatozoa characteristics (recovery rate) determined by comparing the sperm characteristics pre-freezing (SCPF) and frozen-thawing sperm characteristics (FTSC) use the formula described by Khalifa et al. (2016):
Recovery rate = FTSC / SCPF × 100
Statistical Analysis
One-way analysis of variance (ANOVA) was carried out using IBM SPSS Statistics version 22. When ANOVA revealed a significant treatment effect, the values were compared using the Duncan’s Multiple Range Test of the same SPSS program. All values are presented as mean ± standard error (SE), and differences among means were considered significant at P < 0.05.
Table 2: Semen characteristics as affected by the different types of extenders up to 4 hours of incubation at 37 ºC or 4 days of storage at 5 ºC
| Items | Experimental groups |
P value |
||||
| Natural antioxidant groups | Synthetic antioxidant groups | Control group | ||||
| G1 | G2 | G3 | G4 | G5 | ||
| Up to 4 hours of incubation at 37 °C | ||||||
| MOT, % |
85.60±1.10a |
82.40±0.77b |
77.60±0.43c |
76.40±0.43c |
68.20±0.50d |
0.007 |
| LS, % |
89.68±1.09a |
85.84±0.68b |
81.84±0.29c |
78.76±0.88c |
73.36±0.70d |
0.001 |
| AB, % |
7.22±0.56d |
14.43±0.73c |
18.41±0.63c |
23.94±0.98b |
28.93±1.17a |
0.001 |
| IA, % |
89.33±0.40a |
80.50±0.41a |
82.44±0.69a |
81.37±0.71a |
77.83±0.59b |
0.001 |
| PN, score |
7.45±0.06a |
7.21±0.15a |
6.58±0.17b |
6.55±0.16b |
5.74±0.12c |
0.001 |
| Up to 4 days of storage at 5 °C | ||||||
| MOT, % |
82.21±0.52a |
78.00±0.65b |
72.05±0.99c |
70.08±0.79c |
66.21±1.02d |
0.005 |
| LS, % |
87.10±0.56a |
84.43±0.57ab |
78.13±0.94c |
75.25±0.87c |
70.60±0.74d |
0.001 |
| AB, % |
12.72±0.80d |
19.48±1.05c |
20.08±0.86c |
25.60±1.02b |
30.20±0.80a |
0.001 |
| IA, % |
71.28±1.15a |
70.16±0.99a |
70.60±0.17a |
69.12±1.19a |
66.08±1.05b |
0.001 |
| PN, score |
6.79±0.16a |
6.32±0.19a |
5.57±0.19bc |
5.10±0.17c |
4.16±0.11d |
0.001 |
a, b, c and d, means with different superscripts, within each row are significantly different (P < 0.05).
MOT= motility; LS= Live spermatozoa; AB= Abnormality; IA= Intact acrosome; PN= Penetration values;
G1& G2: Containing natural antioxidant genesis of rosemary and ginseng extracts, G3 & G4: Containing synthetic L-carnitine and vitamin E and G5: Free from any type of antibiotics and served as control.
Table 3: Enzymatic activity (U/109 spermatozoa), LPX (µmol/L ) and GSH (µmol/L ) as affected by the different types of ram semen extenders post-equilibration and post-thawing
| Items | Experimental groups |
P value |
||||
| Natural antioxidant groups | Synthetic antioxidant groups | Control group | ||||
| G1 | G2 | G3 | G4 | G5 | ||
| Post-equilibration | ||||||
| ALT |
14.98±0.49c |
16.33±0.42bc |
17.65±0.55b |
19.28±0.57a |
20.29±0.71a |
0.01 |
| AST |
31.35±0.66c |
32.57±0.70c |
33.58±0.72c |
36.37±0.87b |
40.03±0.98a |
0.001 |
| ALP |
82.59±1.54c |
84.01±1.65c |
86.92±1.73bc |
90.55±2.22ab |
95.37±2.90a |
0.005 |
| LDH | 213.30±5.06 | 214.72±5.16 | 217.63±5.21 | 221.26±5.71 | 226.08±6.35 | 0.478 |
| LPX |
1.00±0.20c |
1.20±0.15bc |
1.88±0.23b |
2.49±0.18a |
2.85±0.14a |
0.0001 |
| GSH |
135.17±0.95a |
130.00±0.46a |
129.18±1.05ab |
123.30±0.98b |
113.39±4.26c |
0.0001 |
| Post-thawing | ||||||
| ALT |
16.40±0.41e |
19.22±0.4d |
22.95±0.4c |
35.20±0.4b |
48.50±0.7a |
0.001 |
| AST |
35.65±0.52e |
35.55±0.73d |
40.00±0.99c |
58.53±0.77b |
71.25±1.00a |
0.001 |
| ALP |
85.55±0.6e |
87.4±0.5d |
90.8±0.9c |
97.4±1.1b |
126.0±2.1a |
0.001 |
| LDH |
187.0±0.6e |
217.4±0.5d |
235.8±0.9c |
302.4±1.1b |
386.0±2.1a |
0.001 |
|
LPX |
1.85±0.25c |
2.02±0.20c |
2.85±.02b |
3.12±0.19a |
3.45±0.14a |
0.0001 |
| GSH |
174.55±0.65a |
158.24±0.55ab |
149.22±0.88b |
133.18±1.10b |
109.89±1.16c |
0.0001 |
a, b, c, d and e, means with different superscripts, within each row are significantly different (P < 0.05).
ALT= Alanine aminotransferase; AST= Aspartate aminotransferase; ALP= Alkaline phosphatase; LDH= Lactic dehydrogenase; LPX= Lipid peroxidation; GSH= reduced glutathione; G1& G2: Containing natural antioxidant genesis of rosemary and ginseng extracts, G3 & G4: Containing synthetic L-carnitine and vitamin E and G5: Free from any type of antibiotics and served as control.
Results
Effect on the preservation of unfrozen-semen
A summary of the analysis of variance for incubated or preserved semen is presented in Table (2). Results showed that G1, G2, G3, G4 and G5 significantly (P < 0.001) affect semen characteristics during incubation at 37 °C for up to 4 hours and storage at 5°C for up to 4 days. Semen characteristics observed better values in natural antioxidant groups (G1 & G2) followed by synthetic antioxidant groups (G3 & G4) than control (G5) extender during preservation condition. The prolongation of incubation at 37 °C and storage at 5 °C was increased sperm activity during the first hours of incubation and first days of stored conditions. Subsequently, sperm characteristics measurements declined drastically from two up to 4 hours during incubation and from 2 up to 4 days of storage.
Effect on cryopreservation of frozen semen
Figure (2) shows the effect of cryopreservation procedures within experimental groups on sperm characteristics during post-equilibration (PE), post-thawing (PT) and recovery rate (RR). It will be observed that semen diluted in G1 and G2 under freezing steps gave the highest (P < 0.001) sperm measurements throughout PE, PT and RR compared with G5. In the current study, there were insignificant (P > 0.05) values between semen characteristics such as motility (MOT), live spermatozoa (LS) and intact acrosome through PE, PT and RR at freezing steps between G2 and G3. Then, the sperm values such as MOT was 84 & 83 %; 51 & 50 %; 60.7 & 60.2, LS reached to 88.5 & 86 %; 60 & 55 %; 67.8 & 64, and , AI showed 91 & 90 %; 77 & 75 %; 84.6 & 83.3 when ram semen extended stored PE, PT and RR, respectively.
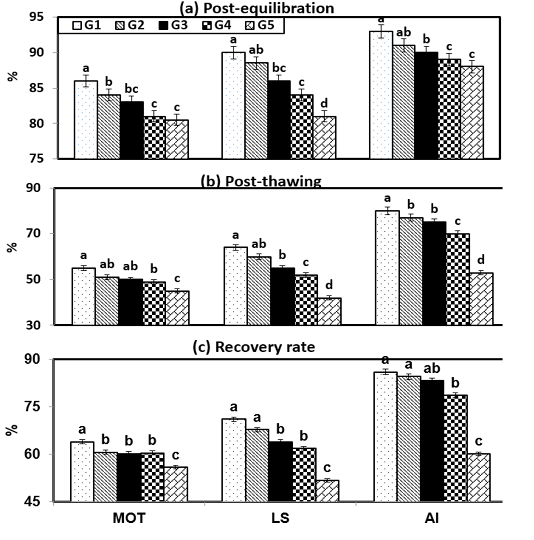
Figure 2: Semen characteristics during cryopreservation of Ossimi ram spermatozoa as affected by the different types of extenders.
Also, sperm penetration ability (PN) and abnormal spermatozoa (AB) post-equilibration and post-thawing are presented in Figure (3). The PN into ewe cervical mucus was significantly (P < 0.001) better with treated extenders especially G1 & G2 and it was in the same trend of the most semen characteristics (MOT, LS & IA), while, the opposite trend of AB was obtained. However, incubation or storage time significantly (P < 0.01) decreased the penetration score in all extended samples.
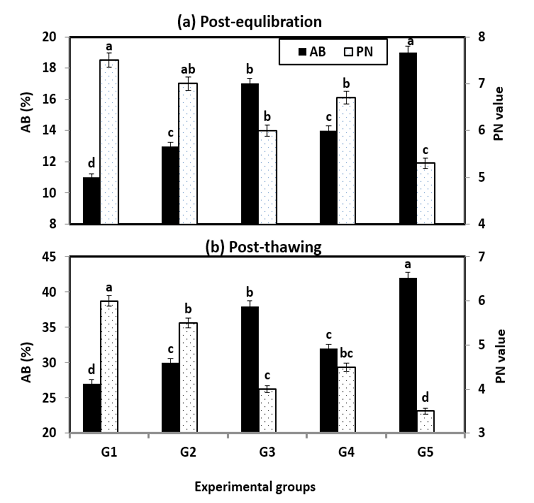
Figure 3: Abnormality and penetration value during cryopreservation of Ossimi ram spermatozoa as affected by the different types of extenders.
a, b, c, d and e, means with different superscripts, within each row are significantly different (P < 0.05).
AB= Abnormality; PN= Penetration value; G1& G2: Containing natural antioxidant genesis of rosemary and ginseng extracts, G3 & G4: Containing synthetic L-carnitine and vitamin E and G5: Free from any type of antibiotics and served as control.
Effect on enzymatic activities
The obtained data in Table (3) indicates the effect of rosemary and ginseng extracts as natural antioxidant or LC, and VE as synthetic antioxidant on enzymatic activities (ALT, AST, ALP & LDH) of ram diluted semen post-equilibration and post-thawing.
Results showed that treated extenders especially G1 & G2 significantly (P < 0.01) lowered the release of ALT, AST, and ALP enzymes into the extracellular medium followed by synthetic antioxidant (G3 & G4) than the control post-equilibration and post-thawing. However, LDH in seminal plasma of diluted ram semen no significant (P > 0.05) differed post-equilibration among experimental diluents but its value deceased significantly (P < 0.001) in treated diluents post-thawing.
Effect on oxidative and antioxidant status
The effect of G1, G2, G3, & G4 on oxidative status of LPX and antioxidant status of GSH of ram diluted semen post-equilibration, and post-thawing are represented in Table (3).
Results showed that there was a significant (P < 0.001) increased in GSH value of G1 & G2, followed by G3 and G4 compared to G5 (control). Also, it can be noted that the supplementation of aqueous extract from rosemary or ginseng was sharply increased the activity of GSH followed by LC and VE (percentage change = 19.21, 14.64, 13.93 & 8.74 post-equilibration; 239.66, 156.90, 65.52 & 43.10 post-thawing) of G1, G2, G3, & G4, respectively relative to control group. Contrary, these supplementation in ram diluent semen significantly (P < 0.001) decreased LPX levels (percentage change = 185, 149, 88 & 20 post-equilibration; 86.49, 68.65, 54.05 & 9.19 post-thawing) relative to control group.
Discussion
The use of natural origin antioxidants rather than synthetic antioxidants in semen preservation is intriguing as of late. In an endeavor to determine a plant-based antioxidant substance, this investigation was conducted to evaluate the effectiveness of aqueous extract of rosemary or ginseng in safeguarding sperm from abundant oxidants.
The current results observed that G1 and G2 were the best extenders, followed by G3 and G4, while the poorest sperm parameter values were obtained in G5 under incubation or refrigeration conditions. From the experimental study, natural antioxidant extenders exhibited the highest ability to sustain sperm motility and viability. This could be attributed to this extender’s capability to supply protection from cold shock, nutrients afford to sperm cells and inhibit microbial growth and goodness physiologic osmotic pressure. Thence, the lowest in sperm parameters through the advancement of preservation time could be attributed to the gradual consumption of nutrients required for sperm metabolic through incubated and stored (Olurode and Ajala, 2016). Besides, the effect of peroxidation comes from polyunsaturated fatty acids in ram sperm cytoplasm membrane, leading to lost cytoplasm membrane, decreased sperm motility, and inhibitor of fructolysis and respiration (Albiaty et al., 2016).
The current study was conducted to determine the efficacy of rosemary or ginseng aqueous extract on protecting stored ram spermatozoa. Rosemary and ginseng aqueous extracts adjusted in G1 & G2 extenders were provided higher action of the spermatozoa characteristics during incubation, post-equilibration, post-thawing than other extenders contained LC (G3), VE (G4) and control (G5). Motlagh et al. (2014) demonstrated that rosemary aqueous extract could improve chilling and post-thawed ram spermatozoa characteristics. Malo et al. (2011) indicated that the positive effect of the aqueous extract of rosemary on progressive motility and epididymal sperm viability post-cryopreservation in bulls. On the other hand, Ramezaninejad and Mehr (2017) obtained that rosemary extract level at 50.0 µg/ml improves sperm quality such as motility (73.69%), livability (71.95%), integrity membrane (73.69%) and LPX (0.93 nM /10×106 sperm cells) of rooster spermatozoa during storage at 4 ºC up to 48 hours compared to 58.35, 69.08, 70.60% and 1.15 nM at 0.0 µg/ml, respectively. Additionally, rosemary extract is deficient in copper (Tabassomi and Alavi-Shoushtari, 2013), the metal co-factor for different enzymes (like desoxy hydroxide dismutase, amine oxidase, and cytochrome oxide) and involved in dismutation of hydroxylation and oxygenation reactions. However, excess copper may oxidize proteins, lipids (which bind to nucleic acids), boost free radical production, decrease oxidative reactions and glucose utilization that reducing or eliminating sperm motility. Inclusion rosemary extract contains zinc as essential constituents of semen extenders; it has been proved to improve the quality of cryopreserved spermatozoa and give better sperm preservation upon freezing processes. Hence, Binsila et al. (2018) revealed that zinc had higher motility, viability, sperm membrane integrity, acrosome reaction, and lower abnormal morphology and involved in the antioxidant capacity.
The sperm motility is considered one of the basic indices of the semen quality in the fertility of animals (Abdelnour et al., 2019; Abdelnour et al., 2020). Gray et al. (2016) reported that Ginseng-supplemented to sperm reduced universal, progressive motility percentage and protected against chromatin damage. The stimulation of motility by ginseng extract was attributed to the ginsenoside content (Chen et al., 1999). Ginseng can alter some of the protein kinases associated with the acrosome reaction pathway (Ahamed et al., 2001). Ginseng contains over 30 different glycoside ginsenosides insulated from their roots (Park et al., 2002). Safavi et al. (2014) ginseng enhance the nitric oxide (NO) synthesis in the endothelium. Nitric oxide has a protective role as an antioxidant. Indeed, ginsenosides have contained L-arginine, which is the source of NO, appears to be a constitutive NO synthase in sperm motility, capacitation, metabolism, and acrosome reaction (Kim et al., 2009). Therefore, the addition of ginseng extract in extender was advisable to improve sperm parameters in ram semen. Ginseng can be used to boost NO creation in mammalian cells, and ginsenoside Re raises the capitation of human sperm and AR from NO / cGMP / PKG pathways (Friedl et al., 2001; Zhang et al., 2007). Evidence has accumulated another way of estrogen signalling in spermatozoa that contain a membrane oestrogen receptor (MER; also recognized as GPR30) (Ded et al., 2010). these systems within spermatozoa indicate that xenoestrogenic and estrogenic substances can affect spermatozoa function directly or indirectly by altering particular kinase activity, Ca2+ (PKC), or cAMP (PKA) (Ded et al., 2010; Bragado et al., 2010).
The actual mechanism by which aqueous extract from the rosemary or ginseng improves the spermatozoa activity remains unknown and is of considerable interest by itself. But like we thought the mechanism of rosemary or ginseng on improving sperm quality is antioxidant property. We have also noted that the aqueous extract of rosemary or ginseng contains important free radical scrapers that can consequently promote the intracellular antioxidant system, including catalase, glutathione, and glutathione peroxidase (Alvarez et al., 1987). Furthermore, the sustained integrities (as refrigerated and frozen-thawing sperm characteristics) in this study could also be attributed to important components (as phenolic) of rosemary or ginseng necessary for reducing free radical to keep sperm cell survival under freezing conditions. Confirmation, Kashyap et al. (2017) recommended that rosemary extracts have a radical scavenging activity up to 95.1% with approximately 90% of the antioxidant activity attributed to carnosol and carnosic acid. The same author also defined that carnosol at level 100-400 mg/kg has been shown to enhance activity of glutathione transferase and has significant antioxidant activity with antimutagenic activity similar to ascorbic acid.
This L-carnitine potentiating role on buffalo sperm cell motility might be attributing to Its crucial role in the mitochondrial β-oxidation process and the esterification of free fatty acids; these esters are oxidized to acetyl CoA that enters the Krebs cycle resulting in ATPs creation by oxidative phosphorylation (Parikh et al., 2009). Moreover, L-carnitine supplements to ram semen extender can improve its motility, viability, and acrosomal integrity during incubation at 37 °C, preservation at 5 °C and cryopreservation. These results came in harmony with El-Raey et al. (2016), who stated that L-carnitine supplementation to buffalo semen extender significantly enhanced its characteristics and protecting its plasma membrane and mitochondrial functional integrity. This vital role might be attributed to its antioxidant characteristics protecting sperm membranes from toxic ROS. L-carnitine supplements to ram semen extender can improve its in vitro penetration rate. These results might be attributed again to enhanced ATP production beside its role as antioxidant (discussed above). Oxidative deterioration can lead to base degradation, fragmentation of DNA and protein cross-linking (Sharma et al., 2004). Damaged DNA spermatozoa stop functioning to penetrate and fertilize the oocyte (Sun et al., 1997). The oxidative stress is defined as the existence of metabolic and radical constituents or so-called reactive (chlorine, oxygen or nitrogen) species (Elnesr et al., 2019; Elwan et al., 2019a). The primary antioxidant capacity (prevent the formation of ROS and scavenging them) of L-carnitine and its secondary antioxidant capability by restoring polyunsaturated oxidized fatty acids found in membrane phospholipids so protecting living cells plasma membranes (Liu et al., 2004). Moreover, Liu et al. (2002) reported that L-carnitine acted by stabilizing the mitochondrial membrane, protecting the cell from apoptosis and markedly enhanced mitochondrial functions and its general metabolic activity reducing oxidative stress pathways. Furthermore, L-carnitine stimulates the entry into mitochondria of long-chain fatty acids for use as energy and promotes the elimination of short and medium chains fatty acids that remain in mitochondria due to normal and abnormal metabolism and inducing mitochondrial ageing (Arduini, 1992).
The current results reported that vitamin E might be ameliorated sperm characteristics at 37 °C and storage at 5 °C and freezing conditions (post-equilibration and post-thawing). These results are in agreement with Ahmadi Hamedani et al. (2016). Generally, Daramola et al. (2017) and Losano et al. (2018) recorded that vitamin E had reported positive effects of storage sperm parameters. Vitamin E inhibits cell impairs by binding to the free radical and neutralizing its unpaired electron mediated by a tocopheryl-quinone’ formation (Schuh et al., 2004). Vitamin E increases intracellular ATP and decline cell permeability and enzyme inactivation peroxidation (Almeida & Ball, 2005; Breininger et al., 2005). Vitamin E captures and deletes oxygen radicals in the membranes, and the alkoxyl and peroxil radicals, fuel for the peroxidation chain reaction generated during the conversion of lipid hydroperoxides (Aitken and Clarkson, 1988).
The evaluation of seminal plasma’s biochemical components is necessary for semen estimation, as physical semen properties alone are not perfect enough for semen assessment (Mann and Lutwak-Mann, 1981). For instance, ALT, AST, ALP, and LDH are fundamental for metabolic procedures that give energy for sperm motility, viability and fertility (Sirat et al., 1996). Accordingly, these enzymes are evaluated in seminal plasma as markers of sperm quality, as they show sperm harm (Sirat et al., 1996). In addition to that, few investigations focused on the existence of these enzymes in ruminant semen. Okab (2007) showed that the spillage of LDH and ALP in rabbit seminal plasma might reveal a strong relationship with both enzyme release and integrity of the sperm cells and acrosomal harm.
There were significant (P < 0.01) differences in activities of ALT, AST, ALP and LDH enzymes in all tested extenders. Their levels were significantly (P < 0.01) higher in ascending order of G1 and G2 as compared to G3, G4 and G5 extenders post-equilibration and post-thawing. Also, there were significant (P < 0.01) increases in the spillage of alanine transaminase, aspartate transaminase, alkaline phosphatase and lactic dehydrogenase enzymes into the extracellular medium through preservation time. These finding are similar to those obtained by Mohamed (2017) and Mohamed et al. (2019).
The obtained data indicated a negative correlation between enzymes activity and content of extenders of antioxidant supplementation of diluted ram semen. The continued elevation in spillage of ALT, AST, ALP and LDH enzymes in the extracellular medium through preservation may due to the destruction of sperm cellular membrane through preservation (Zeidan et al., 2004). The spoilage of ALP enzyme from sperm cells into the seminal plasma, because of cold shock was elevated significantly (White et al., 1954). Such increment in the enzymes activity post storage might indicate expanding the cell harm, which happened with the preservation process (Zeidan, 1994). In the present study, the release of LDH enzyme into the extracellular medium was significantly elevated during the storage period, which might be attributed to degradation of cytoplasmic droplets and plasma membrane when spermatozoa exposed to cold or osmotic shock. Similar results were found by Dhami et al. (1995) in bulls. LDH plays significant role in viability during the preservation, freezing and metabolic activity of spermatozoa (Stalloup and Hayden, 1960). So, LDH enzyme has been implicated in fertilizing the sperm’s ability and viability (Smith et al., 1957). The ALP is the most dynamic de-phosphorylation enzyme in semen that may be directly related to fertility. Its concentration mirrors the functional condition of the accessories sex glands and sperm metabolic activity (Veerabramhaiah, 2011).
The use of nutritional antioxidants is seen as the key to livestock production improvement (Elwan et al., 2019b). Antioxidant enzymes played a significant part in shielding from the damage caused by free radicals. Besides, MDA plays a key role as a strong LPX marker in the cells. As shown in Table (3), rosemary or ginseng extracts reduced (P < 0.001) serum LPX and increased (P < 0.001) serum glutathione contents. These findings confirmed the antioxidant’s role in protecting sperm parameters throughout cryopreservation (Chaudhary et al., 2018; Khalifa et al., 2018). Rosemary and ginseng aqueous extracts displayed impressive antioxidant activity, and this should be predicted as contained phytochemicals and phenolics that are high-powered antioxidants with free radical scavenging. Thus, the previous observation was clearly with Daramola et al. (2017) who evidenced that Antioxidants correspond in enhancing progressive motility, sperm membrane integrity, acrosome integrity, acrosome reaction, and decrease LPX levels during spermatozoa cryopreservation to improve sperm viability parameters.
Conclusion
Our findings demonstrated that supplementing the Tris-dilution during refrigeration and frozen media with natural antioxidant (rosemary or ginseng aqueous extract), and synthetic antioxidant (L-carnitine, or vitamin E), observed a positive impact on activity; surviving and penetration ability of ram frozen-thawed sperm than extender media free of antioxidant. In particularly, in-extended rosemary treatment improves plasma membrane functions, low LPX concentrations, and a loss of sperm viability deceleration compared to the control extender group pre or post-freezing.
Animal Welfare Statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. All animal procedures were performed in accordance with the standards set forth guidelines for the care and use of experimental animals by the Animal Ethics Committee of Zoology Department, Faculty of Science, Beni-Suef University (Approval number, BSU/FS/2014) with the partnership of Animal Production Research Institute (APRI), Ministry of Agriculture, Egypt.
All animals were kept under the same management and hygienic conditions. All rams are individually placed in pens on semi-ribbed floors fed by diet according to NRC’s recommendations (2007). The based ration offered on 60:40 ratio of concentrate feed mixture to forage (as maize silage), respectively. Rice straws ad libitum and fresh water had free access through the experimental periods. The semen samples were collected twice weekly for three weeks by an artificial vagina. Sperm samples from each ram were analyzed separately to take in consideration the variability of animals. From each male, two ejaculates were collected at 10-15 min and then the samples were pooled and designed to experimental work.
acknowledgements
We are being overwhelmed in all humbleness and gratefulness to acknowledge our depth to all those who have helped us to put these ideas, well above the level of simplicity and into something concrete.
Conflict of interest
The authors declare that there is no conflict of interest in this study.
authors contribution
Mahmoud Yassin Mohamed conceived of the presented idea, planned the experiments, designed the model and the computational framework and analyzed the data and wrote the manuscript with input and support from all authors. Hala Mohamed Diab carried out the experiment, and laboratory analysis. Osama Mohamed Ahmed & Fahim, H.I authors contributed to the final version of the manuscript.
References





