Advances in Animal and Veterinary Sciences
Research Article
Hepatorenal and Testicular Functions Concomitant with Induced Hypo and Hyperprolactinemia in Male Rats
Ahmed Mohamed Aboul-Ela, Khalid Mohi El-Dein Ali Moustafa, Nermeen Atef Helmy, Salah Hosny Salah*
Department of Physiology, Faculty of Veterinary Medicine, Beni Suef University, Egypt.
Abstract | This study aimed to investigate early effects of induced hypo and hyperprolactinemia on hepatorenal and testicular functions in rats. Sixty mature male Albino rats were equally divided into 3 groups; control, hypoprolactinaemic (HypoP) and hyperprolactinaemic (HyperP). Treatments continued for one month then individual blood samples were subjected to estimate prolactin “PRL” and testosterone “T” hormones, liver aspartate aminotransferase “AST”, alanine aminotransferase “ALT” and bilirubin as well as kidney functions and malondialdehyde “MDA”. Testes were weighed to calculate gonadosomatic index (GSI) and individual semen samples were collected for semen evaluation. Tissue samples were obtained for histopathology and transmission electron microscopy (TEM). HypoP or hyperP did not change GSI and kidney functions. hypoP increased AST and ALT while hyperP increased AST, ALT, bilirubin and MDA with drop in T level and increased sperm abnormalities. Histopathology revealed mild degeneration in liver, kidneys and testes of hypoP while hyperP showed moderate degeneration in these organs. By TEM, the liver of hypoP displayed nearly normal structure, while in hyperP liver appeared with bundles of collagen fibers, ITO cells and swelling of mitochondria. Kidneys of hypoP showed mild degeneration while hyperP revealed bundles of collagen fibers periglomerular and swelling of mitochondria. It appears that hypoP has minimal effects on liver functions without affecting kidney and testicular functions. Excess PRL is considered a stressor resulting in elevation of MDA with deleterious influence on liver, kidney and testes functions.
Keywords | Hypoprolactinemia, Hyperprolactinemia, Hepatorenal function, Testicular function, Stressor, Rats.
Received | December 23, 2020; Accepted | January 01, 2021; Published | March 15, 2021
*Correspondence | Salah Hosny Salah, Department of Physiology, Faculty of Veterinary Medicine, Beni Suef University, Egypt; Email: salahhosny75@gmail.com
Citation | Aboul-Ela AM, Moustafa KMEA, Helmy NA, Salah SH (2021). Hepatorenal and testicular functions concomitant with induced hypo and hyperprolactinemia in male rats. Adv. Anim. Vet. Sci. 9(5): 682-688.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.682.688
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Salah is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Prolactin is a pituitary hormone with biological actions not limited to reproduction as it controls over 300 separate activities not related to lactation (Freeman et al., 2000). (Capozzi et al., 2015) recorded that PRL is rapidly released during acute stress in males and has immunomodulatory effects as it stimulates the secretion of ACTH, cortisol, and aldosterone beside induces adrenal hypertrophy. The highest binding tissues to PRL are liver and kidney. PRL has a hepatoprotective role in rodents (Moreno-Carranza et al., 2013). On kidneys, PRL regulates osmoregulation and has a potent natriuretic and diuretic action (Ibarra et al., 2005). On testes, PRL can upregulate the synthesis of T through cognate receptors on Leydig cells but high PRL suppresses GnRH and gonadotropins, leading to hypogonadism with decreased level of T (Gill-Sharma, 2008). Dopamine hormone secreted by hypothalamic arcuate nucleus acts on D2 and D4 receptors of lactotrophs causing inhibition of PRL secretion; a process which is regulated by feedback mechanism (Freeman et al., 2000). Agonists of D2 and D4 receptors such as bromocriptine and cabergoline decrease PRL levels while antagonists as domperidone and haloperidol increase PRL circulating levels (Gill-Sharma, 2008; Johnson, 2012).
Deviations of PRL induce problems in liver, kidneys and testes. Gonzales et al. (2007) showed that the severity of hepatic dysfunction with hypogonadism was directly related to PRL level. Ress et al. (2014) revealed that hyperP was frequently recorded in cases suffering from chronic liver dysfunction with hepatic cirrhosis. Jha and Kannan (2016) recorded a significant correlation between serum PRL levels and bilirubin and AST. Husain (2017) recorded weak kidney functions, uremia and dopamine resistance during hyperP. Cui et al. (2018) and Adam (2019) found hyperP in cases suffering from chronic kidney diseases (CKD) with reduced clearance of PRL. The authors observed a direct correlation between PRL and creatinine content. Laszczynska et al. (2002) stated that hyperP led to Sertoli cell degeneration with apoptotic spermatogonia and spermatocytes. Aleem et al. (2005) in rat showed that excess PRL resulted in inhibition of sperm production and its quality. Also, Hasan (2014) showed that alterations of PRL in rats resulted in bad semen quality.
Material and methods
Animals
Sixty mature male Albino rats (180–200g BW) after 3 weeks acclimatization were equally divided into 3 groups; control, hypoP and hyperP.
Induction of hypo and hyperprolactinemia : Induction of hypoP was carried out using cabergoline (Nostifix, Marcyrl, Egypt) at a dose rate of 0.6 mg/kg BW in ml sesame oil (Cap Pharm, Egypt), orally every third day (Eguchi et al., 1995) while induction of hyperP was done by using haloperidol (Haldol, Janssen, Belgium) at a dose rate of 3.0 mg /kg BW in ml sesame oil orally, daily (Samuel et al., 2013). Each control administered 1ml sesame oil, orally daily. Administration continued for 30 days.
Sampling
Individual blood samples were collected at 9 am to separate sera. Each rat was weighed and immediately after rat sacrifice, the testes were weighed and individual semen samples were collected by maceration of cauda epididymis and vasa deferentia. tissue samples from the liver, kidney and testes were immediately obtained for histopathology and electron microscopy.
Biochemical evaluation
Sera were subjected to estimate some biochemical parameters as mentioned by Young (2001).
-AST and ALT using Diamond Diagnostics kits, Germany.
-Bilirubin (total, direct and indirect) using bilirubin assay kit (MyBiosource, USA).
-Serum urea and creatinine using urea kit (Diamond Diagnostics kits, Germany) and creatinine kit (N-Cal kit, USA).
Hormonal evaluation
Sera were subjected to estimate PRL by enzyme immunoassay kit for rat (Bertin Pharma, France) as outlined by (Freeman et al., 2000) and T (testosterone elisa kit, Cayman Chemical, USA) as outlined by Gower (2010).
Stress evaluation
MDA was estimated using MDA kit (Bio-diagnostic company, Egypt) as outlined by Ohkawa et al. (1979).
Semen evaluation
Immediately after rat sacrifice, the testes were weighed to calculate GSI (Adebiyi, 2013) and individual semen samples were collected by maceration of cauda epididymis and vasa deferentia for semen evaluation (Narayana et al., 2005).
Histopathological examination
Tissue samples from the liver, kidneys and testes were prepared and stained by haematoxylin and eosin and examined according to Jones and Hunt (1983). Another tissue samples from the liver and kidneys were immediately obtained for TEM studies (Bozzol and Russell, 1991). The obtained data were subjected to statistical analysis according to SAS Program (1994).
Results
Biochemical evaluation
HypoP led to significant increase in AST and ALT while hyperP led to marked increase in AST, ALT, bilirubin. Hypo and hyperP did not induce any significant variation in kidney functions.
Hormonal evaluation
HyperP led to drop in T level with no effect in case of HypoP.
Stress evaluation
HyperP led to significant increase in MDA with no effect in case of HypoP.
Semen evaluation
HypoP did not influence semen picture while hyperP increased sperm abnormalities. Hypo and hyperP did not induce any significant variation in GSI.
Histopathological examination
Control group showed normal histological structure of liver consisted of normal central vein and hepatocytes, hepatic cords, which radiate from the central vein towards
Table 1: Serum parameters of hypo and hyperprolactinemic rats (Mean±SE).
|
Treatment Parameters |
Control | Hypo-prolactinemia | Hyper-Prolactinemia |
|
Prolactin ( ng / ml ) |
5.11 ± 0.39 |
2.25 ± 0. 31** |
9.14 ± 0. 73** |
|
AST ( U/L ) |
5.06 ± 0.47 |
12.33 ± 0.84** |
48.33 ± 5.86** |
|
ALT ( U/L ) |
47.39 ± 3.21 |
59.07 ± 2.62* |
69.17 ± 4.02** |
|
Bilirubin (total) ( mg/dl ) |
0.54 ± 0.03 |
0.75 ± 0.14 |
2.11 ± 0.49* |
|
Bilirubin (direct) ( mg/dl ) |
0.12 ± 0.01 |
0.13 ± 0.01 |
0.39 ± 0.04** |
|
Bilirubin (indirect) ( mg/dl ) |
0.42 ± 0.09 |
0.62 ± 0.09 |
1.72 ± 0.21** |
|
Urea ( mg/dl ) |
24.51 ± 1.94 |
28.33 ± 2.18 |
28.66 ± 1.23 |
|
Creatinine ( mg/dl ) |
0.50 ± 0.05 |
0.66 ± 0.09 |
0.63 ± 0.07 |
|
Testosterone ( ng/ml ) |
5.56 ± 0.54 |
6.93 ± 1.55 |
1.93 ± 0.20** |
|
Malondialdehyde ( µM ) |
2.60 ± 0.14 |
2.48 ± 0.28 |
3.80 ± 0.24** |
SE : Standard error.
In the same row, values having superscripts differ significantly from their corresponding control ones at * : P < 0.05 and ** : P < 0.001 as revealed by 2 tailed student « t « test.
Table 2: Spermiogram of hypo and hyperprolactinemic rats (Mean±SE).
|
Treatment Parameters |
Control | Hypo-prolactinemi | Hyper-Prolactinemia |
| Gonadosomatic index |
1.03 ± 0.09 |
0.97 ± 0.07 |
0.95 ± 0.08 |
|
Sperm count (millions / ml) |
22.82 ± 2.19 |
21.01 ± 2.89 |
20.10 ± 2.04 |
|
Individual motility % |
72.08 ± 3.30 |
69.95 ± 4.32 |
68.09 ± 5.35 |
|
Live / dead % |
82.60 ± 3.88 |
77.30 ± 4.39 |
76.20 ± 5.63 |
|
Primary abnormalities % |
2.65 ± 0.25 |
3.25 ± 0.69 |
6.65 ± 1.32** |
|
Secondary abnormalities % |
8.35 ± 1.71 |
11.55 ± 2.39 |
18.10 ± 1.34** |
|
Total abnormalities % |
11.00 ± 1.59 |
14.80 ± 1.89 |
24.75 ± 2.43** |
SE : Standard error.
In the same row, values with superscripts differ significantly from their corresponding control ones at * : P < 0.05 and ** : P < 0.01 as revealed by 2 tailed student “ t “ test.
the periphery, could be detected. Hepatocytes alternated with narrow blood spaces, sinusoids, which are lined with Kupffer cells (Fig. 1A). HypoP induced mild hepatic changes in the form of mild degeneration associated with mild necrotic changes, and focal leuckocytic infiltration associated with proliferation of Van Kupffer cells (Fig. 1B) while hyperP showed moderate hepatic lesions with marked congestion in the central veins and blood vessels of portal area, moderate degenerative changes associated with moderate necrotic changes. Meanwhile, pyknotic changes in some hepatocyes, moderate lymphocytic infiltration and Van Kupffer cell proliferation were (Fig. 1C).On the kidneys, Histopathological examination of the kidney specimens obtained from control rats revealed more or less normal histological structure of cortex and medulla with normal renal corpusels structure (Fig. 2A). hypoP kidney showed mild renal lesions including degenerative changes and necrosis of renal lining epithelium, focal lymphocytic infiltration in the interstitial tissues associated with mild glomerulonephrosis (Fig. 2B) while hyperP showed moderate renal lesions, mainly in the form of moderate degenerative changes associated with nuclear pykosis of the renal lining epithelium. Glomerulonephosis and hypercellularity of the glomerular tufft could be detected. Moreover, congestion in interstitial blood capillaries and glomeruli (Fig. 2C). The testes of control group showed more or less normal histological structure. Seminiferous tubules appeared rounded or oval surrounded by a thin basal lamina. Primary and secondary could be detected, spermatocytes, spermatids and spermatozoa were found. Additionally, interstitial tissues showed normal histology including blood vessels and leydig cells (Fig. 3A). HypoP testes showed mild degeneration in seminiferous tubules compared with the control negative group, together with the presence of minimal of abnormal tubules. Mild degeneration and necrosis of some of spermatogenic cells could be detected mild (Fig. 3B) while hyperP appeared with moderate degenerative changes and necrosis associating with moderate degenerative changes and necrosis of spermatogenic cells, In addition to the presence of higher number of abnormal and atrophic and tubules (Fig. 3C). By TEM, the liver of control group showing the normal ultrastructure of the hepatic cell ,having large vesicular nucleus its chromatin clumped mostly at the periphery, mitochon
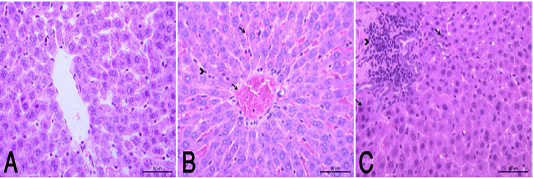
Figure 1 (A): Normal control group showed a normal histological structure of hepatic lobule . (HE X400). (B): HypoP liver showed congested blood vessels including sinusoids (arrow), mild degenerative changes and proliferation of Van Kupffer cells (arrow head). (HE X400) (C): HyperP liver showed necrosis of hepatocytes (arrow) associated with leuckocytic infiltration in the portal area (arrow head), and sever proliferation of Van Kupffer cells (HE X400).
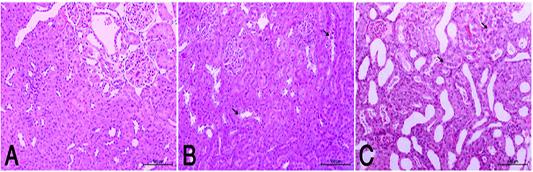
Figure 2 (A): Normal rats showed normal histologic structure of glomeruli (arrow) and renal tubules (arrow head). (HE X200). (B): HypoP kidney showed mild degenerative changes of renal lining epithelium (arrow) (HE X200). (C): HyperP kidney showed moderate degenerative changes associated of lining epithelium (arrow) (HE X200)
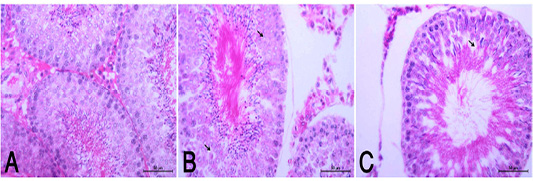
Figure 3: (A): Normal histological appearance of testes in rats of the control group (HE, X400). (B): HypoP testes showing mild degenerative changes (arrow) with minimal distortion of seminiferous tubules (HE, X400). (C): HyperP testes showing degrees of atrophic changes and necrotic changes of spermatogonial cell layer (arrow) with distortion of seminiferous tubules and disorganized germinal epithelia (arrow head) (HE, X400).
dria, rough endoplasmic reticulum, free ribosomes, peroxisomes and inter cellular bile canaliculi having microvilli (Fig. 4A). HypoP liver displayed the hepatic cells nearly of normal morphological appearance and having large vesicular nucleus and cytoplasm containing mitochondria, microbodies, rough endoplasmic reticulum and few small fat globules (Fig. 4B) while hyperP showed bundles of
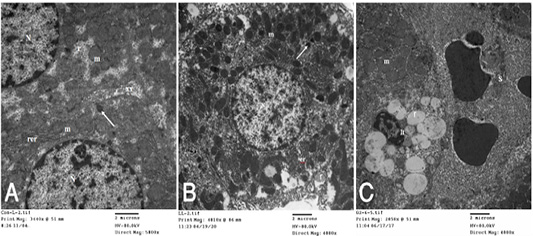
Figure 4: A): Transmission electron (TE) micrograph from liver of control group showing the normal ultrastructure of the hepatic cell ,having large vesicular nucleus (N) its chromatin clumped mostly at the periphery, mitochondria (m),rough endoplasmic reticulum (rer), free ribosomes (r), peroxisomes (arrow) and inter cellular bile canaliculi (xx) having microvilli. (B): T.E. micrograph of liver belonging to hypoprolactinemic group showing the hepatic cells nearly of normal morphological appearance and having large vesicular nucleus (N) and cytoplasm containing mitochondria (m), microbodies (arrow), rough endoplasmic reticulum (er) and few small fat globules (f). (C): T.E. micrograph of liver belonging to hyperprolactinemic group showing congestion of the hepatic sinusoids (S), the Ito cell (It) overloaded with fat globules (f) and swelling of the mitochondria (m) of the hepatic cell.
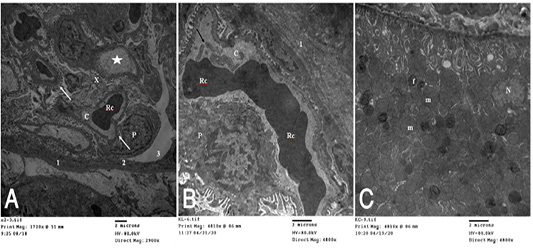
Figure 5 (A): T.E. micrograph of the kidney glomerulus from control group showing the Bawman,s capsule formed by outer thin fibrous layer (1) and inner flat epithelial cells forming the parietal epithelium of the glomerulus (2).The urinary space narrow (3).The capillary tufts (C) contain RBC,s (Rc) and plasma (star) and surrounded by visceral epithelial cells of the glomerulus or podocytes (P) which having primary and secondary processes (arrow). Notice in between presence of small amount of mesangial cell and matrix (X). (B): T.E. micrograph of kidney glomruli belonging to hypoprolactinemic group showing thickening of the fibrous layer of the Bawmen,s capsule (1), swelling of the podocytes (P) and congestion with clumping of the RBCs (Rc) of the capillary tufts (C) with presence of blood platelets (arrow). (C): T.E. micrograph of kidney tubules of hyperprolactinemic group showing marked swelling of the mitochondria (m) with presence of moderate amount of fat globules (f) in the lining epithelium and vesicular nucleus (N).
collagen fibers in perisinusoidal space, congestion of the hepatic sinusoids, the Ito cell overloaded with fat globules and swelling of the mitochondria of the hepatic cell (Fig. 4C). T.E. micrograph of the kidney glomerulus from control group showing the Bawman,s capsule formed by outer thin fibrous layer and inner flat epithelial cells forming the parietal epithelium of the glomerulus. The urinary space narrow. The capillary tufts contain RBC,s and plasma and surrounded by visceral epithelial cells of the glomerulus or podocytes which having primary and secondary processes. Notice in between presence of small amount of mesangial cell and matrix (Fig. 5A). HypoP kidney displayed thickening of the fibrous layer of the Bawmen,s capsule, swelling of the podocytes and congestion with clumping of the RBCs of the capillary tufts with presence of blood platelets (Fig. 5B) while hyperP revealed thickening of the fibrous layer of the Bawmen᾽s capsule with presence of bundles of collagen fibers periglomerular and marked swelling of the mitochondria with presence of moderate amount of fat globules in the lining epithelium (Fig.5 C).
Discussion
The present study discloses that cabergoline led to a significant decrease in serum PRL level, while haloperidol increased PRL level. These results coincide with those of (Gill-Sharma, 2008; Johnson, 2012) who reported the necessity of pituitary D2 and D4 receptors for the dopaminergic inhibitory mechanism.
On the liver, hypoP led to increase AST and ALT without affecting any of the other serum parameters while hyperP led to increase MDA, liver enzymes and bilirubin values as compared with controls. AST and ALT are found in high concentrations in hepatocytes, so liver injury causing destruction of hepatocytes increases both enzymes in the blood but ALT is more specific (McGill, 2016; Kasper et al., 2018). Thus, in the current study elevation of both enzymes in hyperP and to a lesser extent in hypoP reveals incidence of hepatocellular injury. Moreover, the raised bilirubin values in hyperP clarifies the more drastic effect of hyperP to induce hepatobiliary disease (Moyer and Balistreri, 2011; VanWagner and Green, 2015). The aforementioned results are supported by the histopathological and TEM findings as the liver of hypoP group showed mild degenerative changes in hepatocytes while hyperP showed degenerative and necrotic changes of hepatocytes associated with leuckocytic infiltration, proliferation of Van Kupffer cells and congestion of central veins. By TEM, the liver of hypoP appeared of nearly normal structure while hyperP showed liver with vaculations and bundles of collagen fibers perisinusoidal as well as congestion of sinusoids. The hepatic stellate cells (HSC or ITO) were increased overloaded by fat globules and hepatocytes showed swelling of mitochondria. The ITO cells are the main source of activated fibroblasts (Brenner et al., 2012). In liver injury, there is an expansion of ITO cells which differentiate into a myofibroblast-like cell with marked expression of alpha-smooth muscle actin. Myofibroblast-like cells have a high fibrogenic capacity in the diseased liver and are also involved in matrix degradation. Moreover, the transition of ITO cells into myofibroblast-like cells is regulated by an intricate network of intercellular communication between ITO cells and Kupffer cells, damaged hepatocytes, endothelial and inflammatory cells, cytokines and reactive oxygen species (ROS). The ITO cells also plays an active role in a number of liver diseases, with a particular reactivity pattern in fibrotic liver disorders. Moreover, the swollen mitochondria due to hyperP, in the present study, is an indicator that the outer membrane of the mitochondria is going to rupture and the release of the pro-apoptotic proteins such as cytochrome C from mitochondria into the cytosol (Petronilli et al., 2001). Therefore, mitochondrial swelling is a sign of cellular death (Davis and Jeffery, 2002). From the current results, early signs of hyperP in liver are increased enzymes, bilirubin and MDA values besides degeneration of hepatocytes, presence of abundant ITO cells, collagen fibers and swollen mitochondria. The aforementioned results did not appear either in the control or the hypoP. Thus, it could be suggested that hyperP induces liver injury during early stages. In addition, elevated PRL levels may be a causative factor to enhance the activity of ITO cells resulting in liver cirrhosis and induce swollen mitochondria leading to apoptosis. This suggestion receives evidence from the previous findings of a series of studies (Gonzales et al., 2007; Cuifang and Miaoyan, 2009; Jha and Kannan, 2016; Khalil et al., 2017) who recorded elevated plasma PRL levels in males with liver cirrhosis, hypogonadism and portal hypertension. In present results, it appears that neither hypo nor hyperprolactinemia affect serum creatinine and urea. Histopathological examination of hypoP disclosed mild degeneration of renal lining epithelium while hyperP showed moderate degeneration. Under TEM, kidney glomeruli of hypoP illustrated thickening of the fibrous layer of the Bawman’s capsule and swelling of podocytes while hyperP showed presence of bundles of collagen fibers periglomerular, tubular casts, debris and swelling of the mitochondria. Bhatia et al. (2020) reported that swelling of renal mitochondria is associated with decreased mitochondrial membrane potential and is considered a response of increased ROS in renal tissue. Also, mitochondrial dysfunction plays a critical role in the progression of acute and chronic kidney injury. The presence of bundles of collagen fibers periglomerular as well as tubular casts and debris is an indication of glomerulonephrosis (El-Safi and Mohamed, 2017). Therefore, it was investigated, in the current study, that high PRL level is a stressor and elevates serum MDA content. Thus, it could be concluded that high PRL level, although it does not change serum creatinine or urea, but it results in injury-induced oxidative stress and inflammation. Also, the presence of hyperP could be considered as an early sign that the kidney is exposed to stress and going to acute or chronic renal dysfunction.
Results of the current study show that hyperP led to marked drop in serum T; a finding that coincides with that of Grattan et al. (2007) and Henderson et al. (2008) who reported that excess PRL induces infertility in males due to the inhibiting effect on GnRH release and consequently pituitary gonadotropins. Therefore, it could be suggested that excess PRL suppresses GnRH, gonadotropins and Leydig cells to secrete T. HypoP did not influence the spermiogram while hyperP led to increase sperm abnormalities. The histopathological examination disclosed that hyperP appeared with moderate degree of atrophy and necrosis of spermatogonial cell layer as well as distortion of seminiferous tubules and disorganized germinal epithelia. These alterations did not affect GSI. Laszczynska et al. (2002) stated that hyperP associated with apical Sertoli cell cytoplasmic degeneration, increase in percentage of convoluted tubules with apoptotic spermatogonia and spermatocytes as well as abnormalities in reproductive behavior in rodents. MDA result disclosed that high PRL levels could be considered as a stressor. Therefore, the appearance of significant sperm abnormalities could be attributed to the stressor effect of high PRL and drop of T.
Conclusion
It appears from the present study that the early signs of hypoP have minimal effects on liver functions without affecting kidney and testicular ones. On the other side, excess PRL is considered a stressor resulting in elevation of MDA with deleterious influence on liver and testes functions as well as induces tissue damage , So we recommend monitoring the level of prolactin in the blood and treating high cases to avoid the negative effects resulting from it.
Conflict of interest
There is no conflict of interest.
authors contribution
All authors contributed equally to study design methodology, interpretation of results, and writing of the manuscript.
References





