Advances in Animal and Veterinary Sciences
Research Article
Physiological and Hematological Responses of Pregnant Sapera Dairy Goats on Feeding and Drinking Limitation
FA Pamungkas1*, BP Purwanto2, W Manalu3, A Yani4, RG Sianturi5
1Study Program of Animal Production Technology, Graduate School, IPB University, Bogor, Indonesia; 2Vocational College, IPB University, Bogor, Indonesia; 3Department of Anatomy, Physiology and Pharmacology, Faculty of Veterinary Medicine, IPB University, Bogor, Indonesi; 4Department of Animal Production Technology, Faculty of Animal Husbandry, IPB University, Bogor, Indonesia, 5Indonesian Research Institute for Animal Production, Bogor, Indonesia.
Abstract | We conducted the study to evaluate the physiological and hematological responses of pregnant Sapera dairy goats on feeding and drinking limitation. Four pregnant Sapera dairy goats were used in a 4 x 4 Latin square design with four feed regimens: group A feeding was in the form of concentrate and elephant grass silage as much as 1400 gr/day, group B was 1000 gr/day, group C was 700 gr/day, and group D was 500 gr/day. Drinking water was given only for 2 hours from the feeding schedule. The results showed that the physiological and hematological responses of the four groups were in normal condition despite experiencing feeding and drinking limitations. Physiological responses showed differences at several observation points, however pregnant Sapera dairy goat was able to restore thermal balance to the body with no difference in the hematological parameter in the four groups. This shows that the pregnant Sapera dairy goat had adaptive abilities even though it was subjected to feeding and drinking limitation.
Keywords | Goat, Feeding, Drinking, Limitation, Physiological, Hematological
Received | January 27, 2021; Accepted | February 07, 2021; Published | March 15, 2021
*Correspondence | FA Pamungkas, Study Program of Animal Production Technology, Graduate School, IPB University, Bogor, Indonesia; Email: fitrap@yahoo.com
Citation | Pamungkas FA, Purwanto BP, Manalu W, Yani A, Sianturi RG (2021). Physiological and hematological responses of pregnant sapera dairy goats on feeding and drinking limitation. Adv. Anim. Vet. Sci. 9(5): 662-668.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.662.668
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Pamungkas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Increased production needs for livestock in line with the increase in population. Goats are considered to be small ruminants that can adapt to various adverse conditions, particularly those associated with low feed availability during the dry season (Lérias et al., 2014). On the other hand, cultivation of goat especially dairy goat farms requires goats with the ability to produce high milk efficiently. One of the things developed by the Indonesian Research Institute for Animal Production Ciawi Bogor, West Java, Indonesia is starting a crossing program between Ettawah goat females and Saanen goat males with the aim of forming and improved dairy goat breed that will be suitable to be cultivated in the tropics, namely Sapera Goat.
In tropical climates such as Indonesia, quality feeds are only available during the rainy season, while the quantity and quality decrease significantly during the dry season, so that limited feed supply results in a decrease in animal weight or better known as the seasonal load (Lamy et al., 2012). The seasonal load is one of the main problems in animal production especially in tropical climates, where the affected a weight loss of up to 30% (Cardoso & Almeida, 2013). According to Conway et al. (1996), the birth rate in goat only reached 80% due to a decrease in feed intake because the rumen being pushed by the fetus beside of limitation and poor quality of feed.
In addition to feed, drinking water is an important requirement for animal, because its the main solvent for intracellular and extracellular body fluids to maintain heat balance. Casamassima et al. (2008) observed that water limitation also affected other physiological variables such as an increase in several blood metabolites. Compared to other animal, goats are more resistant to the effects of dehydration and reduced dry matter intake (Casamassima et al., 2016). This is possible because the goat has a rumen which acts as a water reservoir (15% of the weight) and can be used during water shortages (Silanikove, 2000). However, lack of water can be a limiting factor on the physiology and productivity of animal (Alamer, 2010).
The physiological parameter of livestock can be used as a reference for assessing physiological responses and a bioclimatic index as a basis for the adaptability of animal to environmental conditions (Wingfield, 2002). Likewise, the hematological parameter can be used as an indicator to evaluate the health status of animal (Piccione et al., 2009), diagnosis of metabolic disease and nutritional status (Soliman, 2014), and even welfare and productivity (Radin et al., 2017). Based on this background, the purpose of this study was to evaluate the physiological and hematological responses of the pregnant Sapera dairy goat on feeding and drinking limitation.
MATERIALS AND METHODS
Ethical Approval
The current study was approved by KomisiKesejahteraanHewanCobaBalitbangtan (KKHB), Ministry of Agriculture Indonesia with regards to its experimentation and the procedures used (Approval no: Balitbangtan/Balitnak/Rm/04/2019).
Study Area
This study was carried out in Indonesian Research Institute for Animal Production which was located at an altitude of 450 to 500 m asl with potential rainfall between 3500 to 4000 mm year-1. The air temperature in the cage ranges from 20.81-31.59 °C with relative humidity of 47.19-99.82% and a wind speed of 1.18-2.02 m/sec.
Sample Collection
This study used 4 pregnant Sapera dairy goats with weight ranges of 35-40 kg (3-4 years old) in 1.6×1.0 m2 individual cages. Four pregnant Sapera dairy goats were used in a 4 x 4 Latin square design with four feed regimens: the group A feeding was in the form of concentrate and elephant grass silage as much as 1400 gr/day (based on pre-research results on the maximum amount of concentrate and silage consumed by pregnant Sapera dairy goat), group B was 1000 gr/day, group C was 700 gr/day, and group D was 500 gr/day. Goats were maintained with twice a day feeding at 07.00 a.m and 15.00 p.m. Drinking water was given only for 2 hours from the feeding schedule. Schedule of feeding and time parameter measurement are presented in Figure 1. Concentrate was in the form of C-Prolac produced by PT. Citra Ina Feedmill Jakarta Indonesia. The nutrient composition of the concentrate was as follow: moisture: 12 %; protein: 18-20 %; fat: 7 %; fiber: 21 %; ash: 10 %; calcium: 0.8-1.0 %, and phosphor: 0.6-1.0 %.
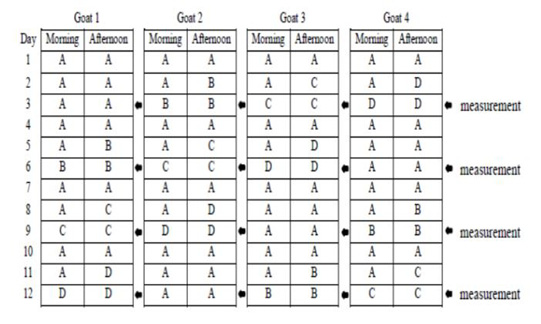
Figure 1: Schedule of feeding and time parameter measurement. Group A feeding was in the form of concentrate and silage as much as 1400 gr/day, group B was 1000 gr/day, group C was 700 gr/day, and group D was 500 gr/day.
Data Collection
Data were collected by measuring physiological parameters starting at 06.00 a.m-18.00 p.m with a measurement time interval every 2 hours, while collection of blood samples for hematological parameters measurement at 06.00 a.m and 18.00 p.m according to the recommendations of the ethics committee related to animal welfare.
Physiological parameters included rectal temperature, skin temperature, body temperature, heart rate, and respiration rate. Rectal temperature (Tr) was measured by inserting the Omron model MC-245 digital thermometer (Omron Healthcare Co. Ltd., Kyoto, Japan) into the rectal up to ± 5 cm. Skin temperature (Ts) was measured with the Omron model MC-720 digital thermometer (Omron Healthcare Co. Ltd., Kyoto, Japan) at four measurement points, namely the brisket (W), chest (X), upper legs (Y), and lower legs (Z). The average skin temperature was calculated based on a modified formula by McLean et al. (1983) as follows: Ts = 0.25 (W+X)+ 0.32 Y + 0.18 Z. According to McLean et al. (1983), body temperature (Tb) was calculated by using the following formula:Tb = 0.86 Tr + 0.14 Ts. Heart rate (Hr) was measured by placing a stethoscope near the left chest for one minute. After that, respiratory rate (Rr) was measured by placing a stethoscope on the chest to calculate inspiration and expiration of breaths for one minute.
Hematological parameters measurement was carried out with blood sampling of goat. 0.5 ml of blood was collected through the jugular vein by first cleaning the neck area with a 70% alcohol swab. Then, a 20 G sterile syringe with a needle depth of 1 inch was inserted into the jugular vein. When the blood came out, the needle was connected to the vacutainer tube at an angle of 45°. After a successful blood collection, the sample was stored in an icebox and then taken to the laboratory for analysis. The hematological parameter profiles were analyzed using the Hematology Analyzer for observed white blood cells (WBC), red blood cells (RBC), lymphocytes (LYM), monocytes (MON), neutrophils (NEU), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width (RDWC).
Statistical Analysis
Data for experiments were analyzed as a replicated 4 × 4 Latin square design with model effects for square, goat within square, period, treatment, and square × treatment using Proc Mixed in SAS (V. 9.1; SAS Institute Inc., Cary, NC, USA) with the model:
Yscit = μ + Ss + Cc(s) + αi + τt + β(χsci) + ατit + τβt(χsci) + ατβit(χsci) + εscit
S = square (1,2,3, 4),
C = goat (1,2,3,4),
α = treatment (1,2,3,4),
τ = time of sampling (1,2,3,4),
β = basal values before treatments,
χsci = Yscit at t = 1, and
εscit = random residual effect.
RESULTS AND DISCUSSION
The physiological response of the pregnant Sapera dairy goats can be seen in Figure 2 and Figure 3. The skin and body temperature of the four groups were statistically insignificant (P>0.05) throughout the day of observation. Skin temperature at 06.00 a.m was 28.1-31.3 °C, then increased until 12.00 a.m (33.9-34.6 °C), then decreased until 18.00 p.m (32.1- 32.5 °C). Likewise, body temperature at 06.00 a.m was 37.0-37.6 °C, then increased until 12.00 a.m (38.2-38.3 °C), then decreased until 18.00 p.m (38.0-38.2 °C).
The skin and body temperature of the four groups showed no significant throughout the day of observation. It shows that feed and drinking water limitation did not influence those parameters, which mean pregnant Sapera dairy goat can keep it within normal ranges. This is in line with a study by Shilja et al.(2016) on Osmanabadi goats where a decrease of feed did not influence skin temperature in several parts of the body (head, scrotum, and stomach).

Figure 2: Conditions of skin, body, and rectal temperature of group A, B, C, and D goats. Within each observation time, bars with different letters (a,b) showed significant differences between groups (P<0.05).
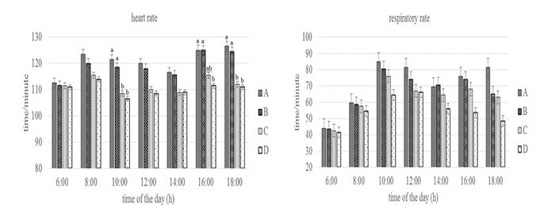
Figure 3: Conditions of heart and respiratory rate of Groups A,B,C, and D goats. Within each observation time, bars with different letters (a,b) showed significant differences between groups (P<0.05).
According to Mengistu et al. (2007), the goat is able to maintain body temperature in normal conditions through the thermoregulation mechanism or the process of maintaining heat balance between production and release. Skin temperature and body temperature at 06.00 a.m increased until 12.00 a.m then decreased until 18.00p.m in line with a study by Piccione et al.(2003) which stated that goat will experience a daily rhythm of body temperature, where the body temperature will increase during the light phase and reach its peak at the beginning or in the middle of the day and then decrease in the dark phase (at night).
The rectal temperature of the four groups throughout the day of observation was between 38.5-39.3 °C. In general, the rectal temperature of the four groups had the same values at several points of observation and only significantly different (P<0.05) at 18.00 p.m especially in Group A and D. The values of rectal temperature were still in the normal range namely 38.5-40.0 °C (Ayo, 1998). The same rectal temperature between groups showed that with feed limitation, the goat maintains internal temperature using specific physiological and behavioral mechanisms (Abdelatif, 2010). Furthermore, Alamer (2010) reported that there was no change in rectal temperature in Aardi goats with drinking water limitations. Whereas the difference in rectal temperature at 18.00 p.m especially in Group A and D was caused by differences in the heat balance mechanism, as indicated by an increase in rectal temperature in group A by 0.2 °C (39.1 to 39.3 °C), while group D decreased by 0.1 °C (39.0 to 38.9 °C). According to Puchala et al.(2007), an increase in rectal temperature due to an increase in heat energy usually occurs about 3-4 hours after feeding.
Heart rate showed statistically significant differences (P<0.05) especially in Group A and D at 10.00 a.m, 16.00 and 18.00 p.m. At the same time, the respiratory rate of the four groups at the beginning of the observation until 16.00 a.m had no statistically differences (P>0.05) but at the end of the observation (18.00p.m) showed differences (P<0.05) especially in Group A and D. Heart rate reflects circulating homeostasis along with general metabolic status (Sejian et al., 2010). The heart rate was above the normal value for the goat that was 70 to 80 times/minute (Dias et al., 2020), but the increase did not show changes in the physiology proven by the equal body and rectal temperature values between groups. Silva & Starling (2003) emphasized the importance of respiratory stabilization, in which an increase in respiratory rate over a long period of time can cause a decrease in blood pressure and CO2 levels, then the heart functions as a means of maintaining normal blood pressure levels by increasing the number of heartbeats. The respiratory rate of the four groups at the end of the observation showed differences in Group A and Group D. This shows that the feed limitation did not make the goat physiologically under stress, the exchange process between the inhaled oxygen from outside the body and the exhaled carbon dioxide is still in a balanced state until 16.00 p.m. The results showed the adaptive ability of the goat to keep the body in normal condition by changing the metabolic activity as reported by Marai et al. (2007).
The body temperature, rectal temperature, heart rate, and respiratory rate of Group D was lower than the other groups. It shows the adaptive ability of Group D in dealing with stress due to feed and drinking water limitations by reducing metabolic activity. Sejian et al. (2018) and Pragna et al. (2018) reported that reduced feed intake can slow down the metabolic process causing the hypofunction of the thyroid gland, thereby preventing additional metabolic heat production can be decrease in body temperature as a form of the adaptive response.This is also possibly due to the thermolability mechanism which leads to a decrease in rectal temperature to overcome feed and drinking water limitation (Wooden & Walsberg, 2002).The decrease in heart rate supported by several studies that reported a correlation between heart rate and metabolic heat production (Popoola et al., 2014). According to Rashid et al. (2013), a reduction in heart rate occurs in cattle with reduced feed intake or reduced activity, or both. The decrease in respiratory rate is due to reduced oxygen consumption and thyroid activity during metabolic processes as a result of reduced feed intake (Umesiobi et al., 2005). Furthermore, Casamassima et al. (2016) reported that reduced respiratory rate due to water limitation is one of the animal defense mechanisms to prevent water loss and dehydration through evaporation that occurs in the lungs.
Conversely, the increase in heart rate in group A was caused by an increase in feed consumption activity. This increase was due to increased blood flow from the core to the surface to facilitate heat loss in the goat (Gupta et al., 2013; Hooda & Upadhyay, 2014), so that heat balance can be maintained (Bernabucci et al., 2010). Mengistu et al.(2007) reported that goat maintains normal body temperature through a thermoregulation mechanism which can be indicated by an increase in heart rate. Increased heart rate will increase blood flow to the heart in response to heat loss due to conduction, convection, radiation, and water diffusion from the skin (Marai et al., 2007; Bernabucci et al., 2010). The increase in respiratory rate especially in Group A is closely related to the activation of the thermoregulatory mechanism due to a high proportion of feeds. The high proportion of feed causes an increase in body heat production so that this heat must be expelled. (Rahardja et al., 2011) reported that increased respiratory rate was closely related to the homeostatic process for dissipating excess heat and maintaining body temperature to return to normal conditions. Furthermore, Kumar et al.(2011) reported that an increase in respiratory rate could be associated with efforts to maintain or restore thermal balance and the process of dissipating body heat through respiration compared to other mechanisms.
The average hematological parameters and the total differentiation of pregnant Sapera dairy goat white blood cells can be seen in Table 1 and 2. The statistical analysis showed no significant differences (P>0.05) between groups on all hematological parameters at 06.00 a.m and 18.00 p.m. This is in line with a physiological response where the pregnant Sapera dairy goat can maintain body temperature to remain within the normal range, a difference in rectal temperature, heart rate, and respiratory rate at a certain time in an effort to maintain the thermal balance due to a decrease in the amount of feed. Changes in hematological values, especially Hb, can be used as the main indicator of various sources of stress (Umesiobi et al., 2005), one of which is nutritional stress due to feeding intake (Sejian et al., 2014). In addition, Hb content plays an important role in pregnancy and fetal survival, especially in the delivery of oxygen to the fetus (Habibu et al., 2014). The insignificantly different Hb values between groups showed the adaptive
Table 1: Hematological parameter of Pregnant Sapera dairy goat during study
| Parameter | Observation | Group | |||
| Time | A | B | C | D | |
RBC (106/µL) | 06.00 | 13.14±1.17a | 13.05±1.48a | 13.04±0.79a | 12.63±1.38a |
| 18.00 | 13.00±0.92a | 12.81±0.95a | 13.02±1.24a | 12.88±0.98a | |
| Hb (g/dL) | 06.00 | 8.40±0.71a | 8.55±0.62a | 8.55±0.47a | 8.33±0.52a |
| 18.00 | 8.43±0.09a | 8.30±0.32a | 8.28±0.61a | 8.25±0.62a | |
| HCT (%) | 06.00 | 22.69±2.29a | 22.49±2.00a | 22.49±1.62a | 21.75±1.91a |
| 18.00 | 22.55±1.00a | 22.13±0.73a | 22.53±1.64a | 22.33±1.23a | |
| MCV (fl) | 06.00 | 17.00±1.41a | 17.25±0.96a | 17.50±1.29a | 17.25±1.71a |
| 18.00 | 17.50±1.29a | 17.25±0.96a | 17.25±0.96a | 17.50±1.91a | |
| MCH (pg) | 06.00 | 6.40±0.49a | 6.60±0.80a | 6.55±0.30a | 6.65±0.40a |
| 18.00 | 6.48±0.41a | 6.53±0.59a | 6.38±0.54a | 6.43±0.54a | |
| MCHC (g/dL) | 06.00 | 36.98±0.64a | 38.18±2.40a | 38.03±1.11a | 38.38±1.63a |
| 18.00 | 37.35±1.91a | 37.50±1.13a | 36.70±0.37a | 36.83±1.54a | |
| RDWC (%) | 06.00 | 34.83±1.96a | 34.13±1.26a | 33.55±1.27a | 35.40±1.63a |
| 18.00 | 34.83±1.91a | 34.75±1.83a | 34.35±1.31a | 34.25±1.22a | |
WBC (103/µL) | 06.00 | 13.74±1.77a | 13.53±1.49a | 14.52±0.66a | 13.31±0.69a |
| 18.00 | 13.97±1.51a | 13.95±2.07a | 13.80±1.03a | 15.78±1.49a | |
A different superscript in the same column for each parameter showed significant differences (P<0.05), RBC: red blood cells; Hb: hemoglobin; HCT: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; RDWC: red blood cell distribution width; WBC: white blood cell.
Table 2: The total differentiation of pregnant Sapera dairy goat white blood cells during the study
| Parameter | Observation | Group | |||
| Time | A | B | C | D | |
LIM (103/µL) | 06.00 | 5.10±1.43a | 5.01±1.88a | 5.67±1.31a | 5.30±0.67a |
| 18.00 | 5.41±1.89a | 5.06±1.13a | 5.37±1.43a | 5.71±1.17a | |
MON (103/µL) | 06.00 | 0.09±0.02a | 0.14±0.04a | 0.13±0.07a | 0.10±0.03a |
| 18.00 | 0.13±0.03a | 0.15±0.04a | 0.12±0.05a | 0.13±0.03a | |
NEU (103/µL) | 06.00 | 8.55±0.91a | 8.38±2.96a | 8.72±1.26a | 7.91±0.72a |
| 18.00 | 8.43±1.15a | 8.74±1.25a | 8.32±1.01a | 9.95±0.97a | |
ability of the pregnant Sapera dairy goat not to stress and to maintain fetal survival despite a decrease in the number of feeds. The hematological values of both RBC, Hb, MCV, MCH, and MCHC were in the range of normal hematological values for goat (Egbe-Nwiyi et al., 2015). This is the same as Red Sokoto goats (Habibu et al. 2014; Habibu et al., 2017) and pregnant Sahel goats (Waziri et al., 2010) in Nigeria during the summer. This shows that the hematological value of pregnant Sapera dairy goat was within the normal values for pregnant goats. The differential values of white blood cells for both LIM, MON, and NEU had no statistical differences (P>0.05) between groups or in line with physiological responses and WBC values, this indicated the adaptive ability of pregnant Sapera dairy goat despite a decrease in the number of feeds. The differential value of white blood cells was in the normal range as reported by (Mbassa & Poulsen, 1993), where the LIM limit value of 2.81-10.97 x103/µL, MON limit value of 0-11 %, and NEU limit value of 1.36-13.5 x103/µL.
CONCLUSION
Although the physiological responses showed differences at several observation points, pregnant Sapera dairy goats were able to restore the body’s thermal balance, as indicated by the absence of differences in the hematological parameters in the four groups. This shows that the pregnant Sapera dairy goat had adaptive abilities even though it was subjected to feeding and drinking limitation.
ACKNOWLEDGEMENT
Gratitude is presented to Ir. AnnekeAnggraeniM.Si., Ph.D. along with the technical staff of the Goat Research Center of the Indonesian Research Institute for Animal Production Ciawi, Bogor, Indonesia for technical assistance. The authors thank the financial support from Indonesian Agency for Agriculture Research and Development (IAARD) Research Funding 2019 and Indonesian Research Institute for Animal Production (IRIAP) with protocol numbers: 1806.201.003.051A/D2/APBN/2019.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
authors contribution
The authors; Fitra A. Pamungkas, Bagus P. Purwanto, Wasmen Manalu, Ahmad Yani and Riasari G. Sianturi contributed to the design and implantation of the research, to the analysis of results and to the writing of the manuscript.
REFERENCES





