Advances in Animal and Veterinary Sciences
Research Article
Impact of Usage Curcuma Longa Extract on Experimental Canine Steroidal Hepatopathy: Clinical and Therapeutic Aspects
Rokia Ahmed1, Shimaa Gouda2, Sabry Mousa1*
1Department of Internal Medicine, Faculty of Veterinary Medicine, Cairo University, Egypt; 2Department of Internal Medicine, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Abstract | Herbal drug (Tumeric) has been reported to have a good effect on body gain and treatment of liver diseases. In addition, this drug acts against the degenerative process of lipid peroxidation which involves free radicals and polyunsaturated fatty acids leading to hepatic pathogenesis. The present study was performed to evaluate the hepatoprotective activity of curcuma longa rhizome ethanolic extract on steroidal induced hepatopathy. Therefore 15 mongrel old dogs were enrolled in this study. Steroid hepatopathy was induced via dexamethasone injection for 14 days followed by administration of curcuma longa rhizome ethanolic extract for two weeks. Clinical, blood samples, ultrasound and cytology examinations were done at day 0, 7 and 14 of induction and day 7 and 14 post curcuma longa rhizome ethanolic extract administration. Haematological analysis revealed a significant decrease in Hb, RBCs in experimental hepatopathic dogs which began to increase at day 7 of treatment by curcuma longa extract, also hepatopathic dogs revealed significant increase in WBCS and after treatment by curcuma longa extract returned to nearly normal value after 7 days of treatment. Serum biochemical analysis revealed significant increase in ALT, AST and ALP in experimental hepatopathic dogs which began to decrease after treatment by curcuma longa ethanolic extract. Ultrasonography revealed increase in echogenicity of liver parenchyma in hepatopathic induced dogs and returned to nearly normal after treatment by curcuma longa ethanolic extract. From the obtained results we concluded that use of curcuma longa extract improve the general health status and weight of the dogs in addition to its great hepatoprotective properties. So it can be recommended as a potent product in protection and treatment of canine hepatopathy.
Keywords | Curcuma longa, Steroidal hepatopathy, Hematological, Biochemical, Ultrasonography, Histopathology.
Received | December 11, 2020; Accepted | December 16, 2020; Published | February 20, 2021
*Correspondence | Sabry Mousa, Department of Internal Medicine, Faculty of Veterinary Medicine, Cairo University, Egypt; Email: sabrymousa769@yahoo.com
Citation | Ahmed R, Gouda S, Mousa S (2021). Impact of usage curcuma longa extract on experimental canine steroidal hepatopathy: clinical and therapeutic aspects. Adv. Anim. Vet. Sci. 9(4): 588-594.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.588.594
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mousa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The liver is the second largest organ in the body which has many biochemical functions as plasma proteins synthesis, catabolism and storage of carbohydrates, degradation of lipids detoxification and excretion of many toxic agents (Kozat and Sepehrizadeh, 2017).
Corticosteroid or glucocorticoid therapy is often used in dog with allergic, anaphylactic, autoimmune diseases and other diseases from 1948. At that moment one type only was discovered. It was cortisone, the synthetic form of the adrenal gland cortex hormone. Unfortunately, during next two years after 1948, a lot of complications were described due to corticosteroids usage. One of the common pathologies in most dogs that were treated with corticosteroids is a steroid hepatopathy which is corticosteroid induced alterations in liver morphological condition (Kondratjeva and Birgele, 2015).
Steroid hepatopathy usually develops after the use of corticsteroids in dogs by 2-3 days. The clinical signs associated with it are lethargy, polydipsia, polyuria, polyphagia, semisoild feces, generalized muscle wasting, rough hair and skin rash (Eman, 2018).
Medicinal plants have very important place as they not only maintain the health but also cure several diseases, including liver disorders without causing any toxicity. Nowadays, more than 50% of all modern drugs in clinical use are of natural products (Govind, 2011).
Curcuma Longa is arizhomatous herbaceous perennial plant of the ginger family zingiberaceae, used in India for thousands of years and a major part of Ayurvedic medicine. It was first used as a dye. Later it was used for its medicinal properties, for treatment for variety of internal disorder as in digestion, throat infection, common cold, liver disorder and anti-inflammatory actions (Savaringal and Lally, 2003). Recently it is used as antibacterial, ant diabetic, antioxidant, anti-inflammatory, anticancer, antiallergic, antiprotozoal and wound healing agent (Singh et al., 2017).
Therefore, the present work focused on investigating the influence of curcuma longa extract use on experimental canine steroidal hepatopathy.
MATERIAL AND METHOD
Animals
Fifteen mongrel dogs (seven female and eight male) weighted (12 - 25 kg) were purchased from laboratory animals research center, faculty of veterinary medicine, Zagazig university. Animals were kept in veterinary teaching hospital at Zagazig University under observation for two weeks before the beginning of the experiment. The protocol of this study has been reviewed and approved by the institutional animals’ care and use committee (IACUC), Cairo University (Vet CU20022020131).
Each dog was housed in a single kennel under condition of natural light and ambient temperature, before beginning the experiment. Each dog was subjected to thorough clinical examination, vaccination against rabies by Biocan R® (inactivated vaccine) and deworming by drontal® plus (one taplet contain 136mg praziquantel, 136mg pyrentel base as pyrantel pamoate, and 680.4 mg febantel) (one tablete/10kg) and sub cutaneous injection of dectomax® (doramectin) (1cm /50kg) and these processes repeated after two weeks. Food was offered twice daily in the form of vegetables as (potato, carrot, zucchini), chicken then cooked together and add bread or cooked rice watering all over the day in metal container.
Experimental protocol
All dogs were exposed to complete comprehensive clinical and physical examinations include (temperature, pulse rate, respiratory rate, and mucous membrane examination). All animals were individually housed 14 days before the start of the study to allow time for acclimatization and application of certain medical percussions.
Whole blood and serum samples were collected from all dogs at day zero at the same time. Ultrasonographical examination was performed for all dogs. Experimental induction of glucocorticoid hepatopathy had been performed for all dogs by I/M injection of dexamethasone® at dose of 1mg/kg for 14 day according to (Filipiak, et al., 2014).
At 7 & 14day after induction whole blood and serum samples were collected from all dogs to estimate selected hematological and biochemical parameters at the same times (7 & 14 day). Ultrasonographical examination was performed for all dogs. Curcuma longa extract were obtained from Herbal plants store called (Haraz) and prepared according to (Salama et al., 2013) and Convert dose from rat to dog according to Paget and Barnes (1964).
Curcuma longa extract was being added to the control diet at dose 0.15 gm/ one kg of food for 14 day.
Selected three dogs were euthanized at 0, 14 day after dexamethasone injection and at day 14 after Curcuma longa extract treatment for histopathological examination according to (Suvarna et al., 2013).
Tools
Blood Sampling technique: Blood samples were collected from cephalic vein of each dog by using hypodermic needle and divided into two parts, first part on EDTA tubes for hematological examination and the second part on plain tube left to coagulate at room temperature for collection of clear non hemolyzed serum for biochemical analysis.
Hematological examination included: (RBCs (10⁶/ml) by using hemocytometer according to method described by (Schalm, 1986), Hb (gm/dl), PCV% by using micro-haematocrit tubes, WBCs (10₃/ml) by using hemocytimeter while differential leucocytic count by examination of thin blood film to apply differential leucocytic count detect percent of neutrophils, esinophills and lymphocytes according to the method described by (Schalm, 1986).
The clear non hemolyzed serum was obtained from tubes by Pasteur pipette and clarified by centrifugation at 3000 rpm for 20 minutes to remove residual red cells, then kept in sterile clean vials at (-20⁰) in deep freeze (AST, ALT, GGT, ALP, total bilirubin, direct bilirubin, total protein, urea, creatinine) (Schalm, 1986).
Samples were collected at day 0, 7 and 14 of dexamethasone administration and day 7 and 14 of herbal administration.
Ultrasonography
Real time ultrasound system with multi frequency probes 3.5, 5 MHZ convex probe and 5, 7.5 MHZ micro convex probe (Pie medical ultrasound imaging system, Toshiba just vision 200 imaging system) and coupling gel were used. Ultrasound examination was done at day 0, 7 and 14 day of dexamethasone injection and at day 7, 14 of herbal extract administration.
Histopathological examination
Selected three dogs were euthanized at 0, 14 day after dexamethasone injection and at day 14 after treatment for histopathological examination. Liver samples were taken in a sterile covered container which contain 10% buffered formalized saline and submitted for cytology and histopathology (Lillile and Fulman, 1976).
Statistical analysis
All data were collected, tabulated and statistically analyzed using SPSS 20.0 for windows (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as the mean ± SD and qualitative data were expressed as absolute frequencies (number) & relative frequencies (percentage). Independent samples Student’s t-test was used to compare between each two groups of normally distributed variables while Mann Whitney U test was used for non- normally distributed variables. One way ANOVA test was for more than two independent groups of normally distributed variables. P-value < 0.05 was considered statistically significant (S), p-value ≥ 0.05 was considered statistically insignificant (NS). Statistical analysis test of normality distribution was carried out according to (Shapiro and Wilk, method 1965). A randomized complete block design with two factors were used for analysis all data with three replications for each parameter. Estimates of LSD were calculated to test the significance of differences among means according to (Snedecor and Cochran, 1994).
RESULTS
General physical and clinical examination
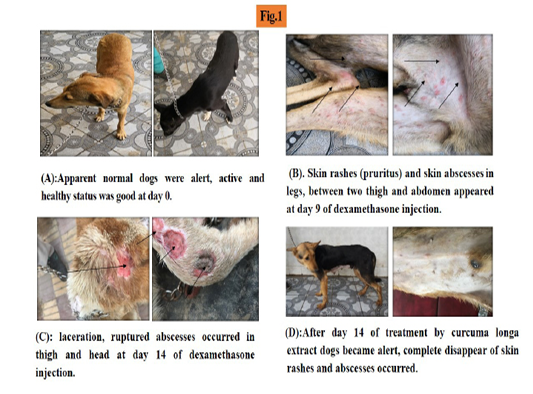
At the beginning of experiment (day zero), the dogs were observed alert, active with normal appetite and weight (Figure 1A).
After one week of induced hepatopathy, the dogs had slight decrease in activity and weight. At day 9, sever lethargy, emaciation, dehydration, skin rashes, alopecia, skin abscess were detected in addition to severe loss in body weight to became about 19 kg (Figure 1B).
At day 14 dogs were seen emaciated, skin laceration and ruptured abscess occurred (Figure 1C).
After treatment by curcuma longa ethanolic extract, the dogs began to improve at day 7 and by day 14; they looked alert with normal appetite, complete disappearance of skin
Table 1: Alteration in haematological parameters all over the experimental study (Steroidal induced hepatopathy for 2 weeks and after administration of curcuma longa extract for 2 weeks) .
|
Hb (gm/dl) |
Pcv (%) |
RBCs (106/μ) |
MCV (fl) |
MCH (pg) |
MCHC (g/dl) |
WBCs (103/μ) |
B (%) |
E (%) |
L (%) |
N (%) |
|
| Control |
15.37a ± 2.90 |
23.97 hi ± 0.37 |
4.13 def ± 0.70 |
58.07g ± .91 |
37.13 ab ± 0.75 |
63.47a ± 1.88 |
10.22 bcd± 2.38 |
0.80 a ± 0.12 |
2.18 a ± 0.29 |
84.5abc ± 1.13 |
17.00 ab ± 2.65 |
|
Dexa (1st week) |
13.83 abc± 3.23 |
25.77 h ± 2.60 |
4.80 bcd ± 1.42 |
86.03ef ± 5.25 |
29.12ef ± 1.64 |
33.87bc ± 1.09 |
16.26 abc± 5.50 |
0.67 ab ±0.15 |
1.48 c ±0.44 |
79.81cd ± 4.27 |
16.00 a-e± 4.36 |
|
Dexa (2nd week) |
10.50 efg ±0.56 |
20.03 j ±2.53 |
2.98 hi ±0.18 |
110.29 b ± 2.17 |
35.21bc ± 0.33 |
31.93bc ±0.45 |
18.58 a ±5.93 |
0.22 d-h ±0.06 |
0.46 fg ±0.13 |
85.03abc ± 2.98 |
12.00 c-g ± 2.00 |
|
Treatment by curcuma longa extract (1st week) |
11.20 def ±0.62 |
33.82 efg ±1.05 |
4.53 cde ±0.31 |
0.01h ± 0.00 |
0.01g ± 0.00 |
33.14bc ±2.09 |
10.33 bcd ±1.15 |
0.20 e-h ±0.04 |
0.55 efg ±0.06 |
78.37d ± 7.86 |
16.83abc ± 2.57 |
|
Treatment by curcuma longa extract (2nd week) |
11.87 c-f ± 1.06 |
35.08d-g ±3.54 |
5.53 ab ±0.23 |
95.54b-f ± 16.46 |
32.16cde ± 4.65 |
33.77bc ±1.32 |
10.05 bcd ±1.83 |
0.18 fgh ±0.08 |
0.68 def ±0.19 |
82.73bcd ± 1.26 |
14.33 a-f ± 2.08 |
N.B:The result with the same letters are non significant while the result with the different letters are significant at P≤0.05
Table 2: Alteration in biochemical parameters all over the experimental study (Steroidal induced hepatopathy for 2weeks and after administration of curcuma longa extract for 2weeks).
|
Total protein (g/dl) |
Albumin (g/dl) |
Globulin (g/dl) |
A/G (ratio) |
ALP (IU/L) |
ALT (IU/L) |
AST (IU/L) |
Total bilirubin (mg/dl) |
Direct bilirubin (mg/dl) |
Creatinin (mg/dl) |
BUN (mg/dl) |
|
| Control |
6.61abc ±2.27 |
2.72cd ±0.73 |
3.89ab ±1.74 |
0.760b ±0.29 |
58.23ghi ±29.12 |
20.20a ±0.53 |
77.08cde ±11.39 |
0.88a ±0.844 |
0.23a ±0.06 |
1.52a ±0.32 |
47.90ab ±15.5 |
|
Dex 1st |
5.78cd ±0.88 |
2.67cde ±0.73 |
3.11abc ±1.37 |
1.015ab ±0.53 |
91.33 e-h ±20.78 |
80.40b ±1.06 |
139.80a ±15.12 |
0.85a ±0.857 |
0.12bcd ±0.02 |
1.60a ±0.30 |
38.51a ±10.66 |
|
Dex 2nd |
5.72cd ±0.92 |
2.64cde ±0.72 |
3.08abc ±1.39 |
1.023ab ±0.55 |
121.73c-f ±32.49 |
120.67c ±1.59 |
150.37a ±10.06 |
0.90a ±0.799 |
0.13bcd ±0.02 |
1.70a ±0.30 |
37.70a-d ±9.42 |
|
Treatment by curcuma longa extract 1st week |
5.5cd ±0.45 |
2.5de ±0.14 |
2.99abc ±0.51 |
0.673b ±0.53 |
42.06hi ±23.33 |
85.33d ±1.18 |
75.47c-f ±25.01 |
0.34ab ±0.216 |
0.13bcd ±0.05 |
0.89b-e ±0.22 |
12.23ef ±2.96 |
|
Treatment by curcuma longa extract 2nd week |
4.71d ±0.53 |
2.08e ±0.07 |
2.63bc ±0.52 |
.813ab ±0.16 |
35.40i ±2.62 |
70e ±2.05 |
70.57d-g ±24.69 |
0.24b ±0.137 |
0.09d ±0.01 |
0.67d-g ±0.07 |
11.25f ±2.14 |
N.B: The result with the similar letters are non significant while the results with the different letters are significant at P≤ 0.05
rashes and abscess. The body weight average improved (Figure 1D).
Haemato-biochemical findings
Hematological alterations are tabulated in (Table 1). The obtained results revealed significant increase in Mean Corpuscular Volume (MCV), total leukocytic count (WBCs) together with significant decrease in Haemoglobin, Paked Cell Volume (PCV), RBCs, Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), Basophilis, Eosinophilis, Lymphocyte and Neutrophils in 2nd week of dexamethasone injection.
After herbal administration for 14 day, there was significant increase and improvement in Hb, PCV, RBCs while there was significant decrease and improvement in MCV and WBCS. Non-significant increase in MCHC, Eosinophilis, Lymphocyte and Neutrophilis was observed in addition to non-significant decrease in Basophils count.
Biochemical finding
As illustrated in Table (2), there was significant decrease in total bilirubin, direct bilirubin and total protein and significant increase in alkaline phosphatase, Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) in 2nd week of dexamethasone injection. There was non-significant decrease in albumin, blood urea nitrogen with non-significant increase in creatinine in 2nd week of dexamethasone injection.
After herbal administration for 14 day, there was significant decrease in total bilirubin, ALP, AST, Creatinine, BUN. Also, there was non-significant decrease in direct bilirubin, total protein and albumin.
Abdominal ultrasonography
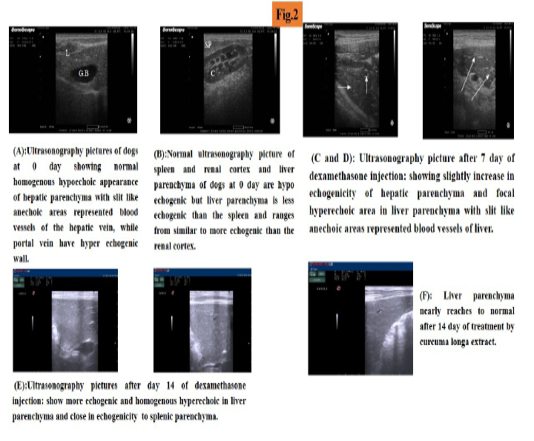
At day zero of experiment, abdominal ultrasound was performed and liver parenchyma was found to be hypoechoic (which is more echogenic than renal cortex and less echogenic than spleen), with slit like anechoic areas representing blood vessels of the liver (hepatic vein without wall and portal veins have hyerechogenic wall) (Figure 2A, B).
At day 7 of experiment liver parenchyma echogenicity increased focal hyperechoic area in liver parenchyma (Figure 2C, D). At day 14 of experiment, increased parenchyma echogenisity than renal cortex and equal to echogenicity of spleen, homogenus hyperechoic liver parenchyma was observed (Figure 2E).
At day 7 of treatment ultrasonogaphical picture showed slight decrease in echogenicity of the liver parenchyma which begin to improve at day 14 of treatment. Liver parenchyma improved more nearly to became normal (Figure 2F).
Histopathological examination
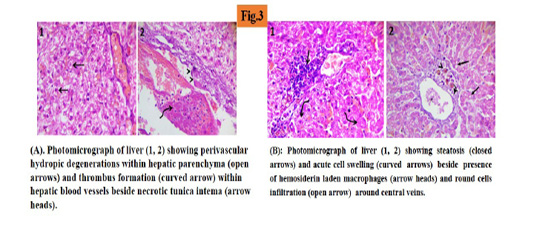
At day 14 of experiment, liver sections showed hydropic degenerations within hepatic parenchyma (45%) and thrombus formation within hepatic blood vessels beside necrotic tunica intema. (Figure 3A).
At day 14 of treatment liver sections showed randomly distributed degenerative changes mainly steatosis (25%) and acute cell swelling beside the presence of hemosiderin laden macrophages and round cells infiltration around the central veins. (Figure 3B).
DISCUSSION
Liver diseases due to many prescription drugs as steroids have become widely used in modern life causing serious health problems (Salama et al., 2013).
Glucocorticoids are used in dogs as anti-inflammatory and immunosuppressive effect but they have serious side effect with long administration period (Khelik et al., 2019).
Curcuma longa has hepatoprotective characteristic similar to silymarin. Curcuma longa has hepatoprotective effects a result of its antioxidant properties which efficacy to maintain the liver status quo, as well as its ability to decrease the formation of pro - inflammatory cytokines. Curcuma longa and curcumin also reserve biliary hyperplasia, fatty changes and necrosis (Salama et al., 2013).
In this study, the observed clinical signs of induced hepatopathy were lethargy, skin lesions such as rough coat, alopecia, skin rashes and localized abcess.These result were in agreement with Gouda, 2010; Abdou et al., 2013 and Eman 2018. After treatment by curcuma longa ethanolic extract lethargy, emaciation and skin lesion diseaper and body weight increases too which go in hand with (Salama et al., 2013; Farjam et al., 2014).
In 2nd week of dexamethasone injection, Hb, RBCs and PCV, were significantly decreased and WBCs was significantly increased. These results were parallel with (Gouda, 2010) but were disagreement with (Eman, 2018) that reported no significant changes in haematological value. Differential leuckcocytic count showed significant decrease in neutrophilis % in 2nd week of dexamethasone injection and these results disagreed with the study of Gouda, in 2010. There were significant decrease in eosinophilis and basophilis in 2nd week of dexamethasone injection and these results were in agreement with the study of Abraham et al. in 2005 and Gouda in 2010.
Regarding our results, it revealed that after treatment with curcuma longa extract there was significant increase in Hb, RBCs and PCV with significant decrease in WBCs.
After treatment with curcuma longa extract, there was significant increase in total protein, albumin and globulin which were in agreement with (Salama et al., 2013).
Our experiment revealed a significant increase in liver enzymes activities in the 2nd week of dexamethasone injection. These results were similar to the previously reported results by (Eman, 2018, Abdou et al., 2013, Gouda, 2010, Khan et al., 2005).
Increase in ALP and AST level may occur as a result of altered membrane permeability and hepatocellular damage.
The elevation of those markers was suggested to be positively linked with the degree of hepatocyte damage (Eman, 2018; Abdou et al., 2013; Hinson et al., 2010). Elevation of ALT level may be due to cellular injury and an increased enzyme synthesis (Ennulat et al., 2010). These enzymes increases in hepatocellular damage which arises from accumulation of glycogen in hepatic cells due to gluconeogenesis (Gouda, 2010).
Farjam et al. (2014) and Salama et al. (2013) reported that, ALP, ALT and AST levels were significantly lower after treatment with curcuma longa extract which go on hand with our results.
Ultrasounic examination at 7 day of induced hepatopathy revealed focal increase in echogenicity of liver parenchyma but diffused increase in echogenicity at 14 day. these results were in agreement with (Foster 2005; Gouda, 2010; Abdou et al., 2013; Nyland et al., 2015; Eman, 2018). Kamel (2008) and Gouda (2010) clarified that, there was increase in echogenisity resulting in marked decrease in clearance portal vein wall that was approved by our research. We added that, after treatment with curcuma longa extract parenchyma echogenicity began to decrease and return to normal.
Histopathological finding revealed that, hydropic degenerations within hepatic parenchyma were (45%) in induced hepatopathy by day 14 and these result were in agreement with (French et al., 2007; Gouda 2010; Eman, 2019). After treatment the liver showed randomly distributed degenerative changes mainly steatosis (25%) and these results were in agreement with (Salama et al., 2013; Farjam et al., 2014).
Conclusion
Our study demonstrated that, Curcuma longa extract had a very good effect on the general health and weight of the animal. Furthermore, it promoted hepatic regeneration and improved the biliary functions as well as its bioavailability. The progression of induced hepatopathy could be reduced and treated using the ethanol extract of curcuma longa rhizomes. The hepatoprotective capability of curcuma longa extract preserved the liver’s status quo in terms of its properties.
Authors Contribution
All authors equally contributed to this article.
conflict of interest
No conflict of interest between authors
REFERENCES