Advances in Animal and Veterinary Sciences
Case Report
The Surgical Intervention for Dirofilaria Infections in One Dog and One Cat
Chattida Panprom1, Ratikorn Bootcha1, Yuthima Kacha1, Soontaree Petchdee2*
1Kasetsart University Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen, Nakorn Pathom, Thailand; 2Department of Large Animal and Wildlife Clinical Science, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen Nakorn Pathom, Thailand.
Abstract | Heartworm disease is one of the most common mosquito-borne diseases in companion animals. Adulticide treatment is recommended for Dirofilaria infection in dogs. However, surgical removal of adult heartworms may be another treatment option for heartworm disease in dogs and cats when the adult heartworms are identified in the right side of the heart such as right atrium, right ventricle , and pulmonary artery. We report the case of one dog and one cat, where a mini-vascular snare was successfully used to remove the adult heartworms from the right ventricle. The dog and the cat had no history of heartworm prevention. The dog presented with a history of coughing, dyspnea, and right hind limb edema, and the cat presented with intermittent cough and vomiting. The patients were evaluated by parasitological investigation and echocardiography. Echocardiography demonstrated the adult heartworms in the right ventricle of the infected dog and cat. The patients underwent the surgical heartworm removal by passing a 4 mm mini-vascular snare through a catheter. The snare wire was guided by fluoroscopy to gently retract the heartworm toward the catheter. Blood analysis after surgical intervention revealed normal limits, including hepatic function. Echocardiography during the follow-up visits did not show complications associated with the intervention procedure, and the cat and dog showed improvements in respiratory signs after the surgical intervention. This study reported the use of a mini-vascular snare to remove adult heartworms in dogs and cats. The snare device offers another treatment option for heartworm disease in companion animals. This technique can immediately remove the adult heartworms and can be used as another treatment option to reduce the complications of the adulticide drugs in dogs. This study suggests that surgical intervention using a mini vascular snare device is not only a safe but efficient therapeutic method for heartworm removal in dogs and cats.
Keywords | Cat, Dog, Heartworm, Mini-vascular snare, Surgical intervention.
Received | December 23, 2020; Accepted | January 01, 2021; Published | February 20, 2021
*Correspondence | Soontaree Petchdee, Department of Large Animal and Wildlife Clinical Science, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen Nakorn Pathom, Thailand; Email: fvetstr@ku.ac.th
Citation | Panprom C, Bootcha R, Kacha Y, Petchdee S (2021). The surgical intervention for dirofilaria infections in one dog and one cat. Adv. Anim. Vet. Sci. 9(4): 571-575.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.571.575
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Petchdee et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Dirofilaria immitis is the most frequent parasite species affecting the lungs and heart in companion animals (Otranto and Deplazes, 2019; Genchi et al., 2011). Microfilariae that living in the heart and pulmonary arteries can be detected in the bloodstream of the infected animals (Dillon et al., 2007). Heartworm may cause respiratory and clinical signs such as cough, dyspnea, vomiting, and weight loss. Respiratory signs are often due to severe pulmonary thromboembolism and inflammatory response of the pulmonary parenchyma and vessels. Microfilaria infection can be observed through morphological identification of circulating microfilaria or specific serological test. Antigen testing is the routine diagnostic method in dogs. However, the laboratory diagnosis of heartworm disease is challenging in cats, as the antigen detection may be insufficient, and the detection of microfilariae in the blood samples of cats is rarely successful and the serological tests can show the negative result because the low number of mature female worm in the blood serum. (Atkins et al., 2000; Genchi et al., 2008). Often a combination of antigen and antibody detection is needed in cats. The presence of adult heartworms in the right ventricle and pulmonary artery can often be imaged in cats by echocardiography (Atkins et al., 2008; Bashir et al., 2014). Adult heartworms in the cardiopulmonary system of the infected animals cause the main changes in the lungs. Infected dogs may develop pulmonary hypertension and a consequence of right-sided congestive heart failure (Dillon et al., 2000). The aim of the treatment is to eliminate the adult heartworms, migrating larvae, and microfilariae, and to reduce the inflammatory reaction caused by dying worms. The American Heartworm Society recommends the use of antibiotics such as doxycycline along with heartworm prevention as the basic method to control heartworm disease in cats. Adulticide therapy in dogs is performed using a protocol with doxycycline and a macrocyclic lactone prior to the three-dose regimen of melarsomine (Grandi et al., 2010; Jones et al., 2014; McTier et al., 2019). Post-adulticide complications need to be considered in all animals, including mainly an increased risk of pulmonary thromboembolism. However, there is currently no satisfactory therapeutic procedure for heartworm infection in cats. Surgical intervention to remove the heartworm may provide an appropriate method to reduce the number of adult heartworms and reduce the reaction from the adulticide drugs as described previously. In this study, we aimed to evaluate the efficacy and safety of mini-vascular snare device for adult heartworm removal in dog and cat.
Materials and Methods
Laboratory investigations
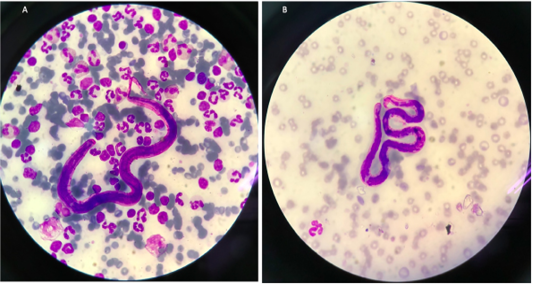
Blood samples were collected and submitted for routine hematological and biochemical investigations. The parasitological investigation included a modified Knott’s test for detecting the circulating microfilariae of the heartworm (Figure 1) and the commercial immune-chromatological rapid tests for detecting heartworm antigens (SNAP 4DX Plus, IDEXX Laboratories, USA.), (González-Miguel et al., 2010; Genchi et al., 2018).
Thoracic radiography
Thoracic radiography was used to diagnose heartworm disease from the characteristics of radiographic images such as changes in the vascular pattern of the pulmonary arteries, and patchy infiltration in the caudal lobe of the lung. The patients were subjected to thoracic image evaluation as shown in Figure 2.
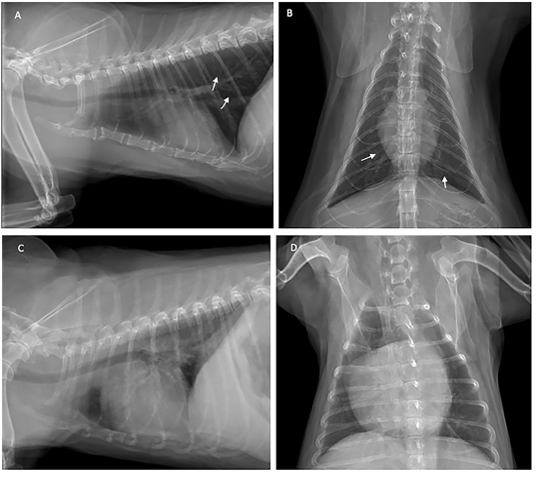
Figure 2: Thoracic radiograph shows the opaque rings of a bronchial pulmonary lung pattern (white arrow) in the cat (A and B), and enlarged heart of the dog (C and D) with heartworm infection.
Echocardiography
The patients (one dog and one cat) underwent a transthoracic echocardiography by using a General Electric Vivid 5s ultrasound system with continuous electrocardiography monitoring to evaluate the cardiac function, worm burden and secondary changes. Echocardiography was performed at baseline, and 1 month after the surgical intervention by one skilled sonographer. The measurement was performed in a parasternal long, short axis and apical four-chamber view in a lateral recumbent position with no sedation. Echocardiographic images were captured and stored for offline analysis. Left ventricular wall structure and function were calculated by measuring the images from two-dimensional and M-mode planes. Ventricular wall thickness and dimensions were recorded during diastole and systole to obtain the parameters such as IVSd; diastolic interventricular septum thickness, IVSs; systolic interventricular septum thickness, LVIDd; left ventricular end diastolic diameter, LVIDs; left ventricular end systolic diameter,
Table 1: Signalment, hematological and serum biochemical parameters before and 1 month after heartworm removal of cat and dog.
| Parameter | Cat | Dog | ||||
| Before | After | Reference value | Before | After | Reference value | |
| Age | 2 years | - | 3 years | - | ||
| Gender | Male | - | Male | - | ||
| Breed | Crossbred | - | Crossbred | - | ||
|
WBC (x103/uL) |
9.98 | 9.96 | 5.5-19 | 20.31 | 10.96 | 6-17 |
|
RBC (x106/uL) |
8.98 | 5.30 | 5-10 | 6.51 | 6.23 | 5-9 |
| HGB (gm%) | 15.4 | 9.3 | 10-15 | 15.50 | 14.30 | 12-18 |
|
PCV (%) |
47.3 | 29.10 | 30-45 | 43.60 | 42.30 | 30-35 |
|
Band neutrophil (x103/uL) |
0.2 | 0.1 | 0-0.3 | - | - | 0-0.3 |
|
Segmented neutrophil (x103/uL) |
7.39 | 7.0 | 2.5-12.5 | 10.15 | 7.56 | 3-11.5 |
|
Lymphocyte (x103/uL) |
1.60 | 1.90 | 1.5-7.0 | 6.90 | 1.97 | 1-4.8 |
|
Monocyte (x103/uL) |
0.1 | 0.4 | 0-8.5 | 3.25 | 0.77 | 0.15-1.35 |
|
Eosinophil (x103/uL) |
0.7 | 0.6 | 0-7.5 | 0 | 0.66 | 0.1-1.25 |
| BUN (mg%) | 25.8 | 19.3 | 15-34 | 20.20 | 21.00 | <34.6 |
| Creatinine (mg%) | 1.98 | 1.82 | <2.0 | 0.97 | 0.34 | <1.8 |
| Total Protein (gm%) | 8.2 | 5.8 | 5.8-7.8 | 9 | 9 | 5.3-7.8 |
| ALT (U/L) | 180 | 108 | 28-76 | 91 | 75 |
<89 |
LVPWd; left ventricular wall diastolic thickness, and LVPWs; left ventricular wall systolic thickness. Right ventricular dimensions and pulmonary artery diameters were observed. Pulmonary velocity was evaluated using a pulse wave Doppler echocardiography, and a right ventricular systolic function was quantified using the fractional area change (FAC) of the right ventricle which was performed in the apical 4 chamber view. The tricuspid annular plane systolic excursion (TAPSE) was measured by placing the M mode cursor through the tricuspid valve in the apical four-chamber view.
Surgical intervention
Surgical intervention was performed using a small pediatric minivascular snare catheter as shown in Figure 3. The study conformed to the guidelines of the Ethical Committee for Animal Experiments, Kasetsart University, Thailand. Patients were premedicated with diazepam (0.5 mg/kg), and then followed by propofol (4 mg/kg) for induction. The anesthesia was maintained by isoflurane at a 2-5% concentration. A 6 French minivascular snare catheter (Merit Medical, USA.) was guided into the right jugular vein and the catheter with the snare was inserted and positioned in the right ventricle under fluoroscopic imaging (Figure 3). Limb-lead electrocardiography and transthoracic echocardiography was used to determine the cardiac rhythm and the position of the adult heart worm (Figure 4). The adult heartworm was removed (Figure 5) from the right ventricle and then retracted to remove the adult heartworm from the heart. After the adult heartworm removal, the guiding catheter was removed, and flushed, and then the skin was sutured with a surgical nylon.
Results and Discussion
The cat showed intermittent cough, and vomiting and the dog presented with a history of coughing, dyspnea and right hind limb edema. Microfilariae were detected in the blood smear samples of the dog and the cat (Figure 1). The antigen detection test was positive in the infected dog, but the antigen test showed a negative result in the cat. Antigen testing is the routine diagnostic method and the most accurate method for detecting of the heartworm infections in the dogs. However, the specific antigen is produced by the adult female uterus, thus, this antigen testing method is not effective for detecting the only male heartworm or an immature of adult heartworm infections, and which may be a limitation of the use of this test in cats. The hematological examination in the cat revealed an increased number of white blood cells characterized by lymphocytosis, and mild monocytosis. The biochemical blood profiles showed increased hepatic enzymes as shown in Table 1. Thoracic radiography of the cat showed a bronchial pattern of pulmonary parenchyma, and opaque rings were visualized on the radiographic image as well as enlarged cranial and caudal lobes of the pulmonary artery (Figure 2). The adult heartworms were detected by echocardiography in both the dog and the cat as a short, segmented strongly hyperechoic parallel lines in the right ventricle as shown in Figure 4. After diagnosis, a surgical treatment protocol using a minivascular snare was designed to remove the heartworm and to stop the progression of the disease. Postsurgical
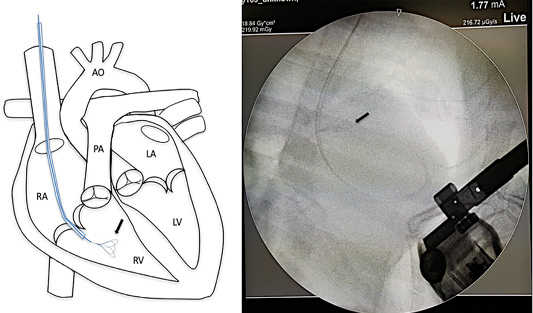
Figure 3: Mini vascular snare device (black arrow) with working space 4-8 millimeter was inserted into the right cardiac chamber with fluoroscopic guidance.
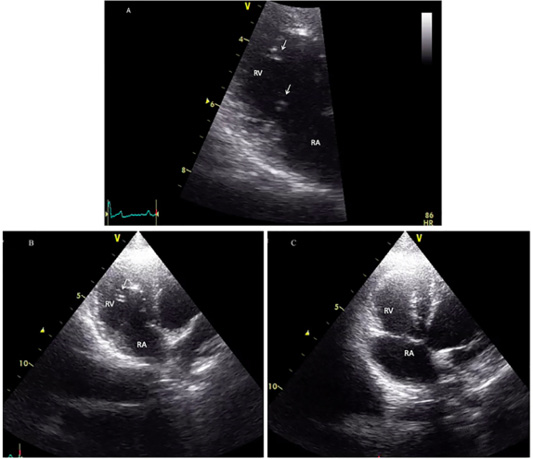
Figure 4: Echocardiographic image showing heartworms (white arrow) in the right ventricle of the infected cat (A and B) and after adult heartworm removal in C. Before the surgical intervention (A and B), heartworms (white arrow) are visible in the right ventricle (RV), no heartworm is visible after the procedure (C).
treatment showed improvements in respiratory signs such as cough and dyspnea (Figure 5B). This surgical procedure can be performed on patients with marked heart enlargement and congestive heart failure, and this is the advantage of this procedure over the conventional adulticide administration in cats. However, there are some limitations of this method, and the use of transcatheter minivascular snares has the limitation of a tortuosity of arteries, such as the branches of the pulmonary artery. Another limitation is that to use the snare device with a fragile substrate such as a heartworm that the heartworm may fragment when retracting the loop of the snare during the closure. In addition, a further administration of anti-inflammatory steroids is necessary to control the inflammation after adult heartworm removal and heartworm prevention is recommended for all infected dogs and cats. However, the cost of heartworm removal procedures is higher than adulticide drug administration. This procedure can reduce the further complications from the administration of adulticide drugs. After the surgical intervention, the cat and the dog showed improvements in the respiratory signs during the follow up visit, and the dog showed improvements in right hind limb edema. Our study provided evidence that the use of a minivascular snare to retrieve adult heartworms is safe and has the potential to control the heartworm infections, and improve clinical signs. This study suggests that the minivascular snare device could be used as a potential surgical treatment for heartworm removal in dogs and cats. Our study also provides a clinical application for the use minivascular snare device to achieve success during surgical intervention in dogs and cats with heartworm disease.
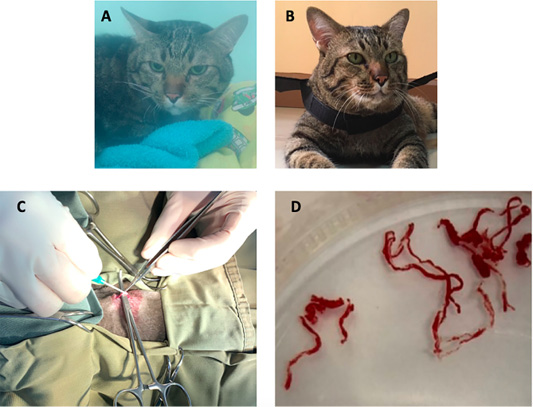
Figure 5: The image showing a cat before surgical intervention (A) in the oxygen chamber, and after adult heartworm removal in (B). The image during the surgical procedure (C), adult heartworms (D) are removed from the right ventricle of the heart.
Acknowledgments
The authors are grateful to Kasetsart Veterinary Teaching Hospital, KamphaengSaen for providing the necessary facilities and Assist. Prof. Dr. Suwarak Wannaratana for providing the vascular snare.
Conflict of interest
The authors declare that there is no conflict of interest.
Author’s contribution
All the authors contributed for the manuscript. Chattida Panprom, Ratikorn Bootcha, Yuthima Kacha, prepared and interpreted the results and Soontaree Petchdee drafted, critical revised and approved a manuscript.
References