Advances in Animal and Veterinary Sciences
Research Article
Molecular Docking Analysis of Curcuminoids from Curcuma longa Extract on iNOS as an Immunomodulator Candidate in Broilers
Herawati Herawati*, Yudit Oktanella, Agri Kaltaria Anisa
Universitas Brawijaya, Indonesia.
Abstract | Enteritis is an inflammatory reaction as a form of the body’s response to the invasion of pathogenic microorganisms in the intestine. However, excessive formation of Nitric Oxide (NO) by inducible Nitrite Oxide Synthase (iNOS) during inflammation processes can trigger cellular toxicity and widespread intestinal tissue damage. Curcuma longa extract contains active compounds, such as curcumin, tannin, alkaloids, flavonoids, and has the potential as an antioxidant, anti-inflammatory, antimicrobial, anti-toxicity, hepatoprotective, nephroprotective, and anticoagulant. This study aimed to investigate the interactions of curcuminoid active compounds in Curcuma rhizome extract on iNOS compared to diclofenac sodium (a non-steroidal anti-inflammatory drug) through computational analysis to explore the potential of Curcuma as a herbal therapy candidate for poultry enteritis. Preparing the three-dimensional structure of iNOS, sodium diclofenac, and curcuminoid active compounds contained in Curcuma extract was the initial step of this study, followed by molecular docking analysis. The results of all molecule interactions were then visualized through Pymol and analyzed by Discovery Studio Visualizer software. The docking visualization results showed that the amino acid residues from iNOS that had the most interactions with ligands were His (421), LYS (261), Trp (203). Molecular docking results of the four active compounds showed that Bisdemethoxycurcumin had the lowest free energy (ΔG) value (-8.00 kcal/mol). The stable conformation location of two ligands (bisdemethoxycurcumin and curcumin) differs from sodium diclofenac, so the type of activity can be said to be a non-competitive inhibition. These results indicated that bisdemethoxycurcumin and curcumin have the potential as anti-inflammatory agents.
Keywords | Enteritis, Poultry, Curcuma, In silico, iNOS
Received | January 18, 2021; Accepted | January 28, 2021; Published | February 20, 2021
*Correspondence | Herawati Herawati. Universitas Brawijaya, Indonesia; Email: herawati58@ub.ac.id
Citation | Herawati H, Oktanella Y, Anisa AK (2021). Molecular docking analysis of curcuminoids from curcuma longa extract on inos as an immunomodulator candidate in broilers. Adv. Anim. Vet. Sci. 9(4): 519-524.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.519.524
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Herawati et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Broiler chickens are meat-producing poultry with a short growth period (Ertanti, 2015) and estimated harvest time on rearing day-35. However, broilers also tend to be susceptible to infectious diseases that attack the body’s immune system (Ulupi et al., 2015), such as an infection of the digestive tract. Contributing stress factors include overcrowding, infectious infections, nutritional imbalances, as well as climate and environmental factors.
Enteritis is a manifestation of gastrointestinal disease caused by microorganisms. It is an inflammation in the intestine that can develop into Necrotic Enteritis (NE), which is a more severe form of inflammation. These lesions can impact poultry’s performance and cause economic losses due to decreased productivity in broilers and layers. Some bacterial infections in poultry can cause enteritis, including Campylobacteriosis, Clostridiosis, and Salmonellosis (Andriani et al., 2012; Coble et al., 2012) with hemorrhagic manifestations in the intestinal mucosa. Inflammation of the digestive tract can occur in the deeper layers, causing conditions ranging from hemorrhagic necrosis of the lamina propria to an abscess in the crypt. In severe inflammation, hemorrhage is found along the digestive tract’s mucosa, causing clinical symptoms of bloody diarrhea.
Proinflammatory cytokines and inflammatory mediators mediate inflammation along the intestine. Nitric Oxide (NO) is an important mediator of the inflammatory process and is known to have antimicrobial activity. NO is endogenously formed by the NO synthase (NOS) enzyme family, by utilizing L-arginine as a substrate and oxygen molecule as well as NADPH as a cofactor. There are three types of NOS isoform, namely: NOS1, neuronal (nNOS); NOS2, inducible (iNOS); and NOS3, endothelial (eNOS). Most NO production is catalyzed by the inducible nitric oxide synthase (iNOS). Increased levels of NO and iNOS in a tissue indicate an inflammatory process (Lukiati et al., 2012). Using immunoperoxidase staining, iNOS was observed to be localized at the gastrointestinal epithelium of individuals with ulcerative colitis, Crohn’s disease, and diverticulitis. This indicates that iNOS plays an important role in the acute mucosal inflammatory response after invasion by pathogenic microorganisms (Mu, 2019). However, an excess amount of NO will be converted into peroxynitrite free radicals (ONOO-), which have a cytotoxic effect. Excessive NO formation by iNOS is directly related to cellular toxicity and tissue damage in animal models of intestinal inflammation (Mu, 2019).
Currently, the utilization of computational screening in the development of drug types has been carried out intensively, fostered by the availability of high throughput datasets and data analysis strategies. The in silico approach demonstrates the ability to produce reliable predictions as well as new knowledge about how drugs works, while helping to reduce the cost of developing drug candidates through in vivo testing. In the in silico test, computer software that has been developed to model pharmacological or physiological processes are used before stepping towards controlled in vitro and in vivo experiments (Chadalawa, 2018).
Anti-inflammatory drugs that are commonly used belongs to the group of Anti-Inflammatory Non-Steroids (NSAIDs), in which sodium diclofenac is one of the examples. NSAIDs work by inhibiting prostaglandin synthesis that blocks the two types of cyclooxygenase, cyclooxygenase I (COX I) and cyclooxygenase II (COX-II). However, NSAIDs also have the potential to cause side effects in the digestive tract, kidneys, and liver (Wilmana et al., 2007). Enteritis in poultry does not always result in specific clinical symptoms. Hence, the objective of a therapeutic approach is to minimize the inflammation so that it does not spread and become chronic. Therapy using NSAIDs in broilers is known to damage the kidneys and liver, and even lead to mortality at high doses (50mg / kg BW) (Palocz et al., 2016). Turmeric (Curcuma longa L.) rhizome is known to have anti-inflammatory, antitumor, antioxidant, anti-arthritis, anti-amyloid, and anti-ischemic activity. This study was aimed to identify the curcuminoid active compounds contained in turmeric that have a potential anti-inflammatory activity using computational analysis approach as the initial step. The ligand-receptor interaction results from the computational analysis will then be used as a reference active compound in the in vitro test. Comprehensively, it was expected that this study would be able to explore the potential of active turmeric compounds as an anti-inflammatory herbal agent for enteritis cases in poultry.
MATERIALS AND METHODS
Equipment
The equipment used is a set of laptops with CORE i-7 processor specifications, 4 GB RAM, and Windows 10 operating system. The software used includes Discovery Studio Visualizer v20.1.0.19295, MGLTools 1.5.6 (Scripps Research Institute) package which consists of Autodock Tools, Autodock Vina, Pymol (DeLano Scientific LLC.), Protein Data Bank (http: // www .rcsb.org / pdb), Uniprot, and PubChem (http://PubChem.ncbi.nlm.nih.gov).
Materials
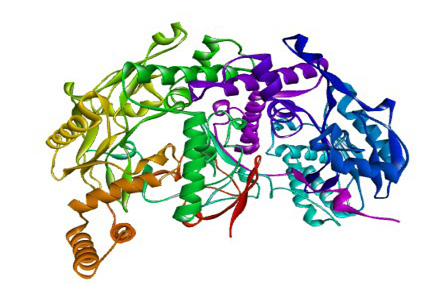
The 3D structure of iNOS was downloaded from the Protein Data Bank in the .pdb format (Figure 1). The 3D structure of the ligand used are the curcumionoid compounds (Figure 3), including: A. Curcumin (E, E) -1, 7-bis (4-Hydroxy-3-methoxyphenyl) -1, 6-heptadiene-3, 5- dione; B: Demethoxycurcumin (1E, 6E) -1- (4-hydroxy-3-methoxyphenyl) -7- (4-hydroxyphenyl) hepta-1, 6- diene-3, 5-dione; C: Bisdemethoxycurcumin (E, E) -1, 7-Bis (4-hydroxyphenyl) -1, 6-heptadiene-3, 5-dione
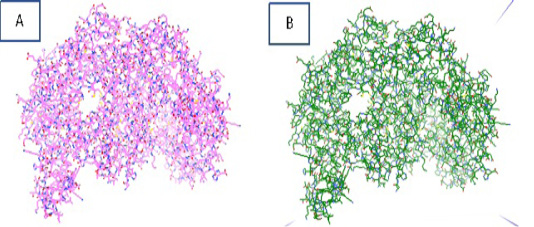
The energy minimization process is carried out using Autodocktools software that produces iNOS protein with an initial energy of 13356.98 kcal/mol, which then becomes 8050.97 kcal/mol. The purpose of protein optimization is to stabilize the structure of the macromolecule and shorten
Table 1: Characteristics of test-ligands
| No | Compound name | PubChem CID | Log P | Molecular weight | Number of hydrogen bond acceptors | Number of hydrogen bond donors | Rotatable bond |
| 1 | curcumin | 969516 | 3.2 |
368.4 g/mol |
6 | 2 | 8 |
| 2 | Demethoxycurcumin | 5469424 | 3.3 | 338.4 g/mol | 5 | 2 | 7 |
| 3 | Bisdemethoxycurcumin | 5315472 | 3.3 | 308.3 g/mol | 4 | 2 | 6 |
| 4 | Sodium diclofenac | 5018304 | 0.7 | 318.1 g/mol | 3 | 1 | 4 |
the time of macromolecular docking with ligands. Figure 2. showed the iNOS protein before and after the optimization process.
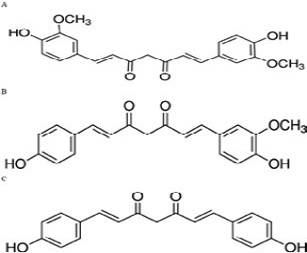
Figure 3: Chemical structure of 3 types of curcuminoids. A: Curcumin; IUPAC name: (E, E) -1, 7-bis (4-Hydroxy-3-methoxyphenyl) -1,6-heptadiene-3,5-dione. B: Demethoxycurcumin; IUPAC name: (1E, 6E) -1- (4-hydroxy-3-methoxyphenyl) -7- (4-hydroxyphenyl) hepta-1, 6- diene-3, 5-dione. C: Bisdemethoxycurcumin; IUPAC name: (E, E) -1, 7-Bis (4-hydroxyphenyl) -1, 6-heptadiene-3, 5-dione (Yang et al., 2015)
Candidate of the test-ligand
In this study, curcuminoid derivatives were used and obtained from the NCBI PubChem database. The results of the test-ligand screening will then be used as inhibitors for the iNOS receptor (Table 1).
Data fromTable 1 showed that all of the test ligands have molecular weights of less than 500 mg/mol, with both the hydrogen bond donor and acceptor values, as well as the log P values meeting the criteria of the Lipinski Rules. All of the test ligands can diffuse through the cell membrane because they have molecular weights of not more than 500 Da (1 Da = 1 g/mol). The number of hydrogen bond donors and acceptors indicates that the higher the hydrogen bond capacity is, the higher the energy that will be needed for the absorption process.
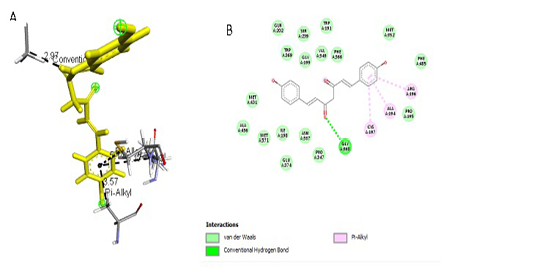
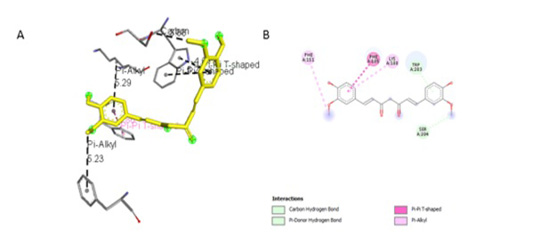
Figure 4:Results of bisdemethoxycurcumin ligand docking to iNOS (A) 3D visualization (B) 2D visualization
Before carrying out the molecular docking simulation, was identifying the number of rotatable bonds to determine the flexibility of the used ligands. The more rotatable bonds are identified, the more flexible the ligands are. Data on the number of ligands and the information of each ligand can be seen in Table 1. A large grid box was used in this study to determine the area of the ligand-receptor docking position so that the ligands can rotate freely to find the most stable position on the receptor. Docking simulation after the determination of the grid box was carried out using AutoDockVina software.
RESULT AND DISCUSSION
Docking of ligands-iNOS receptor
Simulation results of test ligand tethering and comparative ligand obtained bond energy values (ΔG) from the most stable iNOS ligand-receptor interactions (Table 2). Visualization of iNOS ligand-receptor interactions (Figure 4) shows residues from iNOS receptors that play an essen-
Table 2: Results of ligand-iNOS receptor analysis
| Compound (ligand) | Bond energy (ΔG) (kkal/mol) | Distance of hydrogen bonds (Å) | Amino acids that bind | Type of Interaction | The closest residue (<5Å) |
| Bisdemethoxycurcumin | -8.0 |
2,97 3,57 5,14 5,17 |
GLY, 199 ALA, 194 CYS, 197 ARG, 196 |
Hydrogen Pi Alkyl Pi Alkyl Pi Alkyl |
GLY, 199 ALA, 194
|
| Demethoxycurcumin | -4.3 |
3,88 2,36 4,40 4,40 5,25 |
ILE, 497 ASP, 128 LYS, 261 LYS, 261 LYS, 261 |
Pi Sigma Hydrogen Pi Alkyl Pi Alkyl Pi-Pi T-Shaped |
ILE, 497 ASP, 128 LYS, 261 LYS, 261
|
| curcumin | -6,8 |
3,66 4,64 5,27 5,29 4,86 5,23 |
SER, 204 TRP, 203 TRP, 203 Lys, 188 PHE, 185 PHE, 151 |
Hydrogen Pi-Pi T-Shaped Pi-Pi T-Shaped Pi Alkyl Pi-Pi T-Shaped Pi Alkyl |
SER, 204 TRP, 203 PHE, 185
|
| Sodium Diclofenac | -6,7 |
3,44 5,05 3,32 3,32 2,64 4,57 2,73 3,86 3,75 |
GLN, 424 HIS, 421 HIS, 421 HIS, 421 Ala, 404 PRO, 331 Leu, 406 Leu, 398 ARG, 410 |
Hydrogen Pi Alkyl Hydrogen Hydrogen Hydrogen Pi Alkyl Hydrogen Pi Sigma Pi Alkyl |
GLN, 424 HIS, 421 HIS, 421 Ala, 404 PRO, 331 Leu, 406 Leu, 398 ARG, 410
|
tial role in the binding site area (Figure 5 and Figure 6). All ligand-receptor interactions in both the test ligand and the comparative ligand in this study used hydrogen bond distance <5Å.
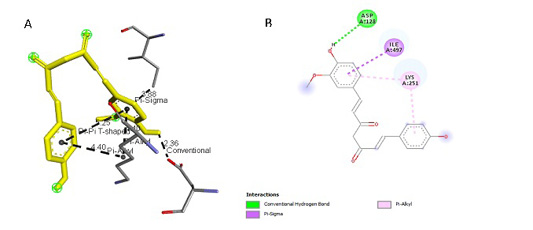
Figure 5: Results of Demethoxycurcumin ligand docking to iNOS (A) 3D visualization (B) 2D visualization
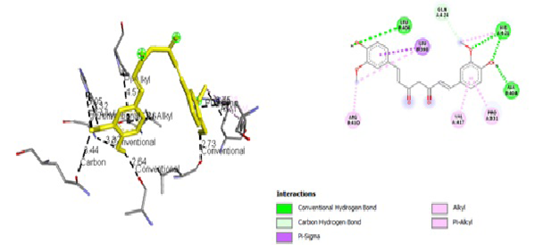
Figure 7: Results of Diclofenac Sodium ligand docking to iNOS (A) 3D visualization (B) 2D visualization
As many as ten poses of ligand-iNOS receptor molecular docking results have been carried out, each with their respective bond energy. The docking models that were chosen had the most negative bond energy values indicating strong interactions. The bond energy and the characteristics of each ligand-iNOS receptor docking results are presented in Table 2.
The observation of amino acid residue contact was aimed to determine any interactions other than the hydrogen bonds between the ligands and target proteins. Amino acid residues that make contact with the ligands have a non-binding interaction between the ligand and target protein, which will increase the affinity and inhibitory activity of iNOS. Based on the visualization of docking results, amino acid residues from iNOS that have the most interactions with ligands are His (421), LYS (261), Trp (203), so that these residues are predicted to play an essential role in the interaction of the compound on the site.
The result of molecular docking is bond energy (ΔG) or affinity, which is a parameter of the conformational stability between the ligand and the iNOS. The difference in the value of the bond energy (ΔG) is due to the difference in ligand binding to the amino acid at the receptor so that the conformation can determine the most stable geometric state of the molecule so that the smaller the bond energy value, the more stable the ligand-receptor interaction will be. Bisdemethoxycurcumin (-8 kcal/mol) and curcumin (-6.8 kcal/mol) have smaller bond energy (ΔG) compared to sodium diclofenac (-6.7 kcal/mol). Table 2 showed that bisdemethoxycurcumin-iNOS has the lowest smaller bond energy (ΔG) value of -8 kcal/mol. These data indicate that the iNOS receptor will be more stable in binding to bisdemethoxycurcumin and curcumin compared to sodium diclofenac.
Based on the number of amino acid residues, sodium diclofenac has the highest amount of residue when compared with the three classes of curcuminoids. The stable conformational location of all curcuminoid ligands is different from sodium diclofenac. This indicates that the type of inhibition by curcuminoid is a non-competitive inhibition.
Research by Syahputra et al. (2014) is in line with the results of this study, which showed that curcumin and bisdemethoxycurcumin have lower affinity energies compared to ibuprofen (an anti-inflammatory drugs) so that it has the potential as an anti-inflammatory agent through the inhibition of the enzyme 12-lipoxygenase. Docking visualization showed that amino acid residues from the 12-lipoxygenase enzyme also have more interactions with ligands, including Leu (311), Lys (310), Trp (647), Glu (226), Leu (233). While in this study, it is known that the amino acid residues of iNOS, which have the most interactions with ligands, are His (421), LYS (261), Trp (203).
Inflammation itself is an indicator that the body’s immune system is fighting against a particular disease. It is a form of body response to destroy, reduce, and localize injury causing-agents as well as injured tissues. The characteristics of acute inflammation include edema, redness, heat, and pain. The acute inflammation process is caused by the release of various kinds of chemical mediators, such as the product of leukocyte cell activity, protease enzymes in plasma, vasoactive amines, and arachidonic acid metabolites.
According to (Boroumand et al., 2018), turmeric’s curcuminoid content can be used as a potent blocker for NF-kB activation. NF-kB regulates inflammatory mediators such as chemokines, cytokines, adhesion molecules, kinases, and enzymes. When the curcuminoid content suppresses NF-kB activation by inhibiting IkBα kinase and AKT, NF-kB products such as iNOS will not be over produced. Research conducted by Olkowski et al. (2006) found that the early-stage of necrotic enteritis (NE) is an inflammatory response followed by an unconventional condition, which leads to a certain degree of inflammatory manifestation.
The prolonged inflammatory process has negative consequences such as exacerbating tissue damage and diverting energy and nutrients digested in the feed for the need to fight the invasion of pathogenic microorganisms, resulting in decreased performance in broilers and layers. Also, the release of pro-inflammatory cytokines causes «malaise» syndrome in poultry that causes pyrexia, anorexia, and weight loss. Because of these negative effects, the inflammatory response needs to be optimized so that livestock productivity can be increased (Tarradas, 2020). This indicates that intestinal inflammation can initiate the development of NE lesions, and treatment/therapy needs to be sought to suppress the inflammatory response, hence decreasing the severity of the lesion caused by NE.
Conclusion
Docking comparison of curcuminoid active compounds with sodium diclofenac (NSAIDs) through in silico using molecular docking techniques on iNOS, which plays a role in inflammatory pathomechanism, showed that bisdemethoxycurcumin and curcumin have lower ΔG values than diclofenac sodium. The stable conformation location of the two ligands differs from sodium diclofenac so that the type of activity can be said to be a non-competitive inhibition. These results indicated that bisdemethoxycurcumin and curcumin compounds have the potential as anti-inflammatory agents. The activity of these two compounds still needs to be further proven by in vitro and in vivo tests.
Conflict of interest
We declare that there are no conflicts of interest associated with this paper, and there are no financial interest related to this research and its outcome. As corresponding author, I confirm that this manuscript has been read and approved by authors member, and the submission has been considered under authors member agreement.
authors contribution
All authors contributed equally.
References