Advances in Animal and Veterinary Sciences
Research Article
Pathological and Immunopathological Studies on Broiler Chicks Infected with Chicken Anemia Virus
Doaa IA Mostafa1*, Rehab I Hamed2, Sanaa M Salem3, Fatma Abdallah4, Hala M N Tolba5
1Department of Clinical Pathology, Animal Health Research Institute, Zagazig branch, Agriculture Research Center (ARC), Egypt; 2Department of Poultry diseases, Reference Laboratory for Veterinary Quality Control on Poultry Production Sharkia Laboratory, AHRI, Agriculture Research Center (ARC) Egypt; 3Department of Pathology, Animal Health Research Institute, Zagazig branch, Agriculture Research Center (ARC), Egypt; 4Department of Virology, Faculty Of Veterinary Medicine, Zagazig University; 5Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt.
Abstract | Chicken anemia virus (CAV) has become a significant worldwide problem in the broiler industry due to its economic losses. The aim of this work was, investigation of the hematological, clinicobiochemical, pathological, and immunohistochemical changes associated with Chicken Anemia Virus (CAV) infection using processed tissue homogenates positive for CAV. CAV isolated from clinically infected broiler chickens (100 samples from 10 flocks) at Sharkia governorate Egypt. Ten samples representing the 10 flocks were done by PCR test to determine the presence of CAV-DNA in tissue samples (spleen, thymus, bone marrow, and bursa fabricius of suspected cases). Supernatant (0.2 ml) of tissue homogenates was inoculated intramuscular into one-day-old chicks; control group received only phosphate-buffered saline. Blood and tissue samples were collected at 7, 14, and 21 days old. Positive presence of CAV-DNA genome in the PCR products (583 bp in size) of amplified CAV-DNA was extracted from the tissues of diseased chicks. The infected group with displayed signs of growth retardation; normocytic normochromic anemia with PCV <22%; significant decreases in the levels of total protein, albumin, globulin and triglycerides that were detected at 7 days post infection and persisted till 21 days, in addition to a significant increase in Alanine aminotransferase and Alkaline phosphatase activities; and decrease in serum calcium level with a significant increase in serum inorganic phosphorus level throughout the experimental period. The deviation in liver enzymes revealed the presence of liver disorder consistent with the histopathological changes demonstrating marked depletion in lymphocyte cells in the bone marrow, thymus and bursa of fabricius with liver damage.
Keywords | Hematology, Bone marrow, Biochemistry, Pathology, Immunofluorescence.
Received | January 19, 2021; Accepted | January 26, 2021; Published | February 20, 2021
*Correspondence | Doaa IA Mostafa. Department of Clinical Pathology, Animal Health Research Institute, Zagazig branch, Agriculture Research Center (ARC), Egypt; Email: Doaaibrahim706@gmail.com
Citation | Mostafa DIA, Hamed RI, Salem SM, Abdallah F, Tolba HMN (2021). Pathological and immunopathological studies on broiler chicks infected with chicken anemia virus. Adv. Anim. Vet. Sci. 9(4): 508-518.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.508.518
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mostafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Chicken anemia virus (CAV) has emerged as an important disease that causes severe anemia and hemorrhage in young chickens. This disease is associated with immunosuppression even in subclinical cases, leading to enhanced infections due to secondary viral and bacterial agents. CAV is a small, non-enveloped, icosahedral virus belong to family Cicoviridae and genus Gyro virus, measuring 25 nm in diameter with a negative-sense, single-stranded circular DNA genome of approximately 2.3 kb pairs (Gelderblom et al., 1989).
In Egypt, few studies are available about the CAV circulating in chicken population (Zaki and El-Sanousi, 1994; Hegazy et al., 2010) but Hussein et al. (2002) previously investigated CAV and they detected a 418 base pair (bp) CAV-specific band in blood and tissue samples of infected broiler chicks. (Hegazy et al., 2014) proved that CAV is widely distributed among chicken flocks at different localities of Sharkia governorate, Egypt. In Egypt, Zaki and El-Sanousi, 1994 reported that the incidence of CAV antibodies in serum samples collected from broiler breeder, layer, and day old broiler flocks was 70%.
Most of the clinical signs of CAV appear at 10–14 days of age. The disease is extremely lethal at this age and causes anemia, bone marrow hemorrhages, and atrophy of the cells in the bone marrow and thymus in young chicks (Adair, 2000). It is clinically characterized by anorexia, weakness, unthriftiness, weight loss, severe anemia (paleness of comb, wattle, eyelids, and legs), and sudden death (Kuscu and Gurel, 2008). The lesions manifest transient severe bone marrow aplasia and pancytopenia (Umar et al., 2014).
Older birds infected with chicken anemia virus (CAV) do not show clinical signs, but immunosuppression is observed in the form of poor response to vaccine with reduced resistance to other secondary infections producing significant effects on the growth rate and profitability of the flock (Adair, 2000) and like other immunosuppressive diseases as IBD Arafat et al. (2017); Arafat et al. (2020); H9N2 Arafat et al. (2018), Eladl et al. (2019a), Eladl et al. (2019b), Awadin et al. (2019), Awadin et al., (2020), Eladl et al. (2020) and Cryptosporidiosis Eladl et al. (2014). Clinical infection with CAV can be abolished through vaccination of breeders (maternal antibodies) during the first 3 weeks of age (Yuasa et al., 1980; Otaki et al., 1992). Moreover through age-related resistance (neutralizing antibodies) in chickens older than 3 weeks of age (Rosenberger and Cloud, 1989b; Hu et al., 1993).The maternal antibodies persist until about 3 weeks of age (Otaki et al., 1992; McNulty, 1998). CAV infections can be diagnosed by detecting the viral antigen, viral DNA, or virus-specific antibodies (McNulty, 1998).
CAV occurs in young chicks before 3weeks of age or in older immunosuppressed chickens. Susceptible chicks develop a severe anemia (Taniguchi et al., 1982) and immunosuppression that may lead to secondary infections (VonBulow and Schat, 1997). Chicks experimentally infected at one day of age were found to develop lesions at eight days post-inoculation (p.i.) and anemia at 12–16 days p.i. (VonBulow and Schat, 1997).
Histologically, there is complete atrophy of lymphoid cells in a wide variety of tissues. In the bone marrow, cells of all hematopoietic lineages are depleted during active infection (Smyth et al., 1993). Most of the chicks completely recovered by 32–36 days p.i., when neutralizing antibodies are present (VonBulow and Schat, 1997).
Therefore, the objective of the present study was advanced diagnosis of chicken anemia virus infection in broiler chicken and investigation of its clinical signs and hematological, clinicobiochemical, pathological, and immunofluorecent changes.
Materials and methods
Field sample
For obtaining the virus, tissue and blood samples were collected from chicks at different ages (from 1 to 21 days) and located in Sharkia Governorate, Egypt during late 2018 and 2019, suspected to be infected with CAV. They displayed paleness, stunted growth, depression, and mortalities. A total of 100 samples from 10 different flocks were collected from the liver, spleen, thymus, bursa of Fabricius, and bone marrow under aseptic conditions and were stored at −70°C for DNA extraction and genome detection by PCR and preparation of tissue extract. 10 samples representing the 10 flocks were done by PCR test to determine the presence of CAV-DNA. In addition, fresh blood samples on EDTA anticoagulant were used to detect PCV as described by (Coles, 1986).
Identification of CAV-DNA by PCR
Nucleic acid extraction: The extract from tissue suspension was performed using the QIAampminielute virus spin kit (Qiagen, Germany, GmbH). The nucleic acid was stored at −70°C to be used. Oligonucleotide primers were supplied by Metabion, Germany, and are listed in Table (1). Then PCR amplification, the primers were used in a 25-µl reaction in an Applied Biosystem 2720 thermal cycler. The primary denaturation step was performed at 95°C for 5 min, followed by 35 cycles of 94°C for 30 s, 56°C for 40 s, and 72°C for 45 s. A final extension step was performed at 72°C for 10 min. (Hegazy et al., 2010).
Gene ruler: a ladder of 100–600 bp was used (Qiagen Germany).
The PCR test was applied at RLQP, Animal Health Research Institute, Dokki, Egypt.
Preparation of Tissues extract
Collected tissue samples were ground with a mortar and pestle in PBS with addition of antibiotic mixture (1000 I.U. penicillin / ml + 1 mg streptomycin sulphate/ ml) to prepare a 20% tissue homogenate. The tissue homogenate was centrifuged at 3000 rpm for 20 min. The supernatant was stored at –70oC until used for inoculation (Zhou et al., 1997).
Experimental chicks and design
The study was conducted at the Animal Health Research
Table 1: Primer sequences, target genes, and amplicon sizes
| Target gene |
Primer sequence (5′–3′) |
Length of amplified product (bp) | Reference |
| VP-1 | AAT GAA CGC TCT CCA AGA AG |
583 bp
|
|
| AGC GGA TAG TCA TAG TAG AT |
Institute, Zagazig branch, and faculty of veterinary medicine, Zagazig University, Sharkia, Egypt. A total of 60 SPF broiler chicks aged one day were obtained from Kom Oshim Fayoum farm divided into two equal groups, each consisting of 30 birds. The first group was control negative that was neither infected nor vaccinated and received only phosphate-buffered saline at the same time. The second group of chicks were inoculated I/M with positive CAV tissue homogenate 20% at a dose of 0.2 ml per bird at the age of one day (Hussein et al., 2002). The chicks were housed under standard hygienic environmental conditions until the end of the experiment (21 day old), with continuous lightening being provided throughout the experiment. Food and water were allowed ad libitum.
Sample collection
Blood samples: Two blood samples from five chicks in each group were collected at 7, 14, and 21 days from the wing vein under a septic conditions using a sterilized syringe. The first blood sample was collected on EDTA anticoagulant for hematological examination. The second blood sample was collected without the anticoagulant for the separation of serum and stored at freezer −20°C for further biochemical analysis.
Bone marrow sample: Bone marrow smears were prepared by collecting fresh marrow from the femur of broiler chickens at the time of necropsy for microscopic examination.
Tissue samples: Specimens were collected from thymus, bursa of Fabricius, spleen, liver, and bone marrow at 7, 14, and 21 days post infection in 10% formalin for pathological and immunohistochemical examinations. Specimens were examined macroscopically then fixed in 10% neutral formalin and embedded in paraffin. Sections measuring 5 µm thickness were prepared, stained by hematoxylin and eosin as described by Suvarna et al. (2013), and then examined microscopically.
Hematological studies
Erythrocyte counts were recorded using an improved Neubauer hemocytometer and Natt and Herrick solution as a special diluent for chicken blood as described by Harrison and Harrison, (1986). The packed cell volume was estimated using microhematocrit centrifuge as described by Coles, (1986). Hemoglobin level was estimated using the cyanomethemoglobin colorimetric method after centrifugation according to Van Kampen and Zijlstra, (1983). Mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were calculated.
Bone marrow smear
Slides were prepared after collecting fresh marrow from the femur of broiler chickens. The prepared slides were left for air drying, fixed using methyl alcohol, stained by Giemsa, and then examined microscopically (Feldman et al., 2000).
Biochemical studies
Total protein (TP) level was determined as described by Krohn, (2005). Albumin level was determined according to Pinnell and Northam, (1978). Serum globulin level was calculated by subtracting the obtained albumin level from the TP level. The serum triglyceride concentration was measured according to Mamoru et al. (1977). The activities of serum ALT , ALP, calcium, and inorganic phosphorus were determined as described by Tietz, (1995).
Immunofluorescence
A tissue isolated from the bone marrow, spleen, thymus, liver, and bursa was fixed and processed for paraffin-embedding, cut into sections measuring 5 µm thickness and then placed on a coated slide and completed according to the method previously described by Maria and Hansen, (2003). The indirect immunofluresence antibody test was applied at poultry diseases and Research department, Animal Health Research Institute, Dokki, Egypt, using a fluorescein-labeled antibody to rabbit IgG (H+L) produced in goat [catalog No. 172-1506 (KPL)].
Statistical analysis
The data in this study were statistically analyzed by a T-test according to Tamhane and Dunlop, (2000) using the MSTAT-C computer program. Results are presented as mean ± SE, and the statistical significance was set at (P≤0.05).
Results
Clinical investigation of field sample of CAV infected chicks
The chicks of field samples were showed depression, stunted growth, generalized weakness. The PCV of their blood samples were between 17-24% which suspected to be infected with CAV.
Field investigation of CAV in broiler chicken using PCR
The analysis of DNA extracted from pool of tissues from each case of diseased flocks (thymus, bursa of Fabricius, liver, and spleen) by agarose gel electrophoresis yielded positive PCR products (583 bp) as shown in Figure 1.
Results of the experiment
Clinical and necropsy findings: Clinical examination of the CAV-infected group (G 2) revealed signs of anorexia, depression, generalized weakness, pale comb and wattles, stunting, growth retardation, and droopy appearance. The mortality rate was about 10%. The necropsy findings consisted of markedly atrophied thymus glands, subcutaneous hemorrhages and yellow fatty bone marrow. Atrophied bursa of fabricius, enlarged pale liver and spleen in addition to retardation of keel bone ossification also observed (Fig. 2).
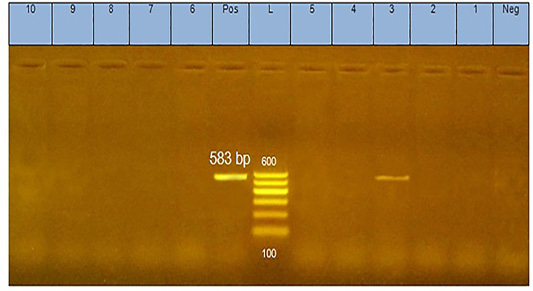
Figure 1: PCR products (583 bp in size) of amplified CAV-DNA extracted from the tissues of diseased chicks. Lane 3 is positive demonstrating the production of specific ampliconat 583bp; Lanes1, 2, 4, 5, 6,7, 8,9, and 10 are negative.
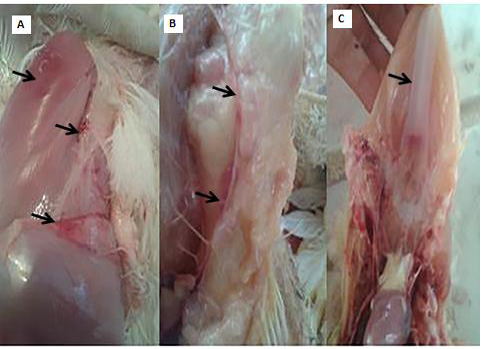
Figure 2: PM lesions of the CAV-infected group showing subcutaneous hemorrhages(A), thymic atrophy(B), and retardation of keel bone ossification(C).
Hematological findings: The CAV-infected group manifested signs of normocytic normochromic anemia at 14 days of age, which were represented by a significant decrease in RBC count and Hb% and a severe decrease in PCV to <22% in all samples along with non-significant changes in MCV and MCHC that persisted till 21 days of age (Table 2).
Bone marrow smears findings: Microscopically, the bone marrow smear of CAV-infected chicks (G 2) demonstrated depletion of erythrocytes, thrombocytes, and granulocytes and their precursor cells (Fig. 3A) at 7 days of age. These findings became more severe at 14 days of age till the disappearance of blood cell precursors and replacement with adipose tissue (Fig. 3B and C). At 21 days old they start to improve and show some increase in the blood cell precursor (Fig. 3D).
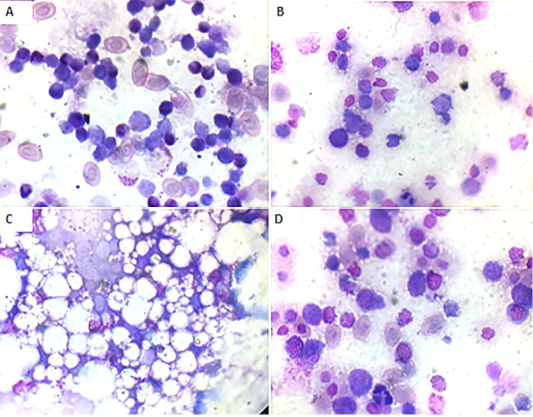
Figure 3: Bone marrow smear of the CAV-infected group, Giemsa stain X oil emersion lense: (A) 7 days post infection showing depletion of blood cells and their precursors.(B) 14 days post infection showing severe depletion of blood cells and their precursors. (C) 14 days post infection showing replacement with fat cells. (D) 21 days post infection showing increase in blood cells and their precursors.
Biochemical changes: There were significant decreases in TP, albumin, globulin, and TG levels from the age of 7 days that persisted till 21 days of age, in addition to a significant increase in ALT and ALP activities, in the CAV-infected group. There was also a significant decrease in serum Ca levels with a significant increase in serum inorganic phosphorus levels at 7 days old then decrease till the end of the experiment (Table 3).
Histopathological changes: Liver sections of the CAV-infected group at 7 days of age revealed multifocal periportal inflammatory cell infiltrations, primarily lymphocytic aggregations, mild congestion, and very widening blood vess-
Table 2: Hemogram of the tested groups at different experimental time periods (mean±SE) (n=5).
|
Time
Parameters |
7 days old | 14 days old | 21 days old | |||
| Control | CA | Control | CA | Control | CA | |
|
RBC count (×106cells/μl) |
2.78 ± .06 |
2.63 ± 0.05* |
2.9 ± 0.09 |
1.89 ± 0.04* |
2.86 ± 0.09 |
2.09 ± 0.11* |
|
Hemoglobin (gm%) |
10.43 ± 0.3 |
9.55 ± 0.16* |
10.66 ± .017 |
7.1 ± 0.22* |
10.5 ± 0.2 |
8.33 ± 0.09* |
| PCV(%) |
32.23 ± 0.55 |
28.69 ± 0.92* |
32 ± 0.58 |
20.8 ± 0.72* |
31.51 ± 0.61 |
22.3 ± 0.46* |
| MCV (fl) |
116.1 ± 4.69 |
109.08 ± 1.96 |
110.7 ± 1.6 |
110.45 ± 5.49 |
110.26 ± 1.38 |
106.85 ± 3.44 |
| MCH (pg) |
41.5 ± 5.45 |
36.31 ± 0.40 |
36.82 ± 0.82 |
38.12 ± 1.35 |
36.74 ± 0.55 |
39.97 ± 1.59 |
| MCHC (%) |
33.28 ± 1.07 |
33.27 ± 0.69 |
33.19 ± 0.21 |
34.51 ± 0.54 |
33.15 ± 0.09 |
37.33 ± 0.95* |
n: number of samples RBCs: Red blood cells PCV: Packed cell volume MCV: Mean corpuscular volume. MCHC: Mean corpuscular hemoglobin concentration
*Significant at P≤0.05
Table 3: Some biochemical parameters of the tested groups at different experimental time periods (mean±SE) (n=5).
|
Time
Parameters |
7 days old | 14 days old | 21 days old | |||
| Control | CA | Control | CA | Control | CA | |
|
Total protein (g/dl) |
3.45 ± 0.22 |
2.51 ± 0.18* |
3.37 ± 0.39 |
2.34 ± 0.21* |
3.77 ± 0.02 |
2.41 ± 0.13* |
| Albumin (g/dl) |
2.17 ± 0.19 |
1.38 ± 0.06* |
2.1 ± 0.33 |
1.37 ± 0.06* |
2.44 ± 0.17 |
1.38 ±0.04* |
| Globulin (g/dl) |
1.28 ± 0.04 |
1.13 ± 0.14 |
1.27 ± .06 |
0.97 ± 0.12* |
1.33 ± 0.03 |
1.05 ±0.12* |
| A/G ratio |
1.69 ± 0.14 |
1.2 ± 0.12 |
1.65 ± 0.16 |
1.4 ± 0.4 |
1.83 ± 0.13 |
1.31 ±0.24* |
| ALT (U/L) |
19.56 ± 1.03 |
29.76 ± 0.82* |
19.41 ± 1.8 |
29.65 ± 0.53* |
20.74 ± 0.98 |
29.11 ± 0.93 |
| ALP (U/L) |
183.3 ± 07.3 |
286 ± 4.9* |
184.82 ±13.05 |
284.00 ± 2.9* |
175.14 ± 6.53 |
288.55 ±7.36* |
| TG (mg/dl) |
266.71 ± 9.24 |
157.50 ± 16.61* |
257.1 ± 7.35 |
173 ± 8.2* |
264.53 ± 3.7 |
197.66 ± 8.1* |
|
Calcium (mg/dl) |
8.59 ± 0.26 |
6.63 ± 0.28* |
8.77 ± 0.31 |
6.75 ± 0.43* |
8.94 ± 0.4 |
8.22 ± 0.31 |
| Inorganic phosphorus(mg/dl) |
4.17 ± 0.10 |
5.38 ± 0.20* |
4.9 ± 0.14 |
3.22 ± 0.06* |
4.37 ± 0.35 |
3.30 ±0.15* |
n: number of samples A/G: Albumin globulin ratio TG: Triglycerides *Significant at P≤0.05
-els as well as sinusoids (Fig. 4A). The thymus section revealed severe depletion of lymphocytes in the majority of lobules as well as in both cortex and medulla with congested interstitial blood vessels (Fig.4B and C). The spleen section demonstrated marked congested blood vessels in addition to extreme depletion of subscapular lymphoid elements that were characterized as empty spaces (Fig. 4D).The bursa of Fabricius section revealed severe dilatation in the majority of follicles as marked empty spaces in the center follicles, which increased due to edematous inter follicular fibrous connective tissues (Fig. 4E). There was a marked increase in the inter follicular fibrous connective tissue followed by necrotic follicular elements in few of the examined sections of the bursa of Fabricius (Fig.4F). The bone marrow section demonstrated a marked decrease of all the hematopoietic series with a marked increase in fat globules that were prominent (Fig.4G and H).
Histological examination of the liver sections of CAV-infected birds after 14 days old showed focal perivascular in
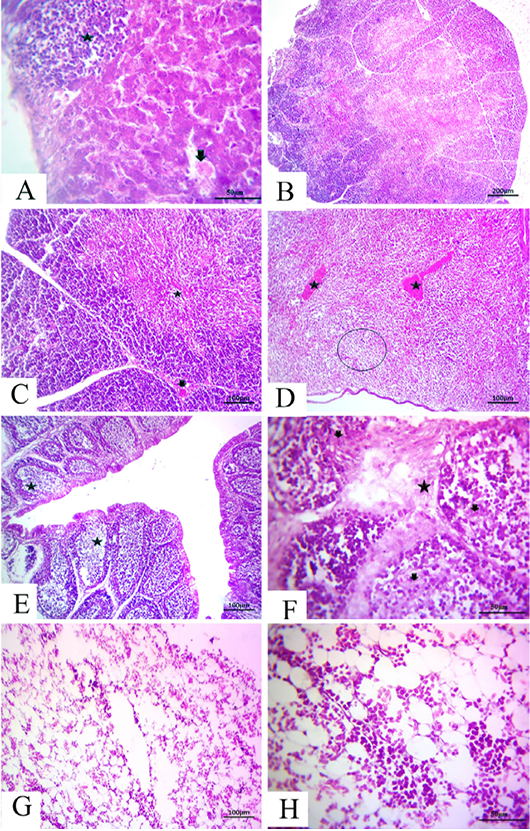
Figure 4: at (7 days old): (A):High magnification of the liver showing focal lymphocytic aggregations(star), widening and congested sinusoids (arrow), H&E×200. (B):Representative photomicrograph of the thymus section showing severe depletion of lymphocytes in the majority of lobules as well as both cortices, H&E×100. (C):Representative photomicrograph of the H&E-stained thymus section showing severe depletion of lymphocytes in the majority of lobules as well as both cortices (star) and medulla with congested interstitial blood vessels (arrow), H&E×100.. (D):Representative photomicrograph of the spleen section showing marked congested blood vessels (stars) subscapular lymphoid elements (circle), H&E×100. (E):Representative photomicrograph of the bursa of Fabricius section showing severe dilatation in the majority of follicles (stars), H&E×100. (F):Representative photomicrograph of the bursa of Fabricius section showing marked increase in interfollicular fibrous connective tissues (star), followed by necrotic follicular elements (arrows), H&E×100. (G): Representative photomicrograph of the bone marrow section showing marked decrease of all the hematopoietic series, H&E×100. (H): High magnification of the bone marrow showing depletion of the majority of hematopoietic series with marked fat globules, H&E×200.
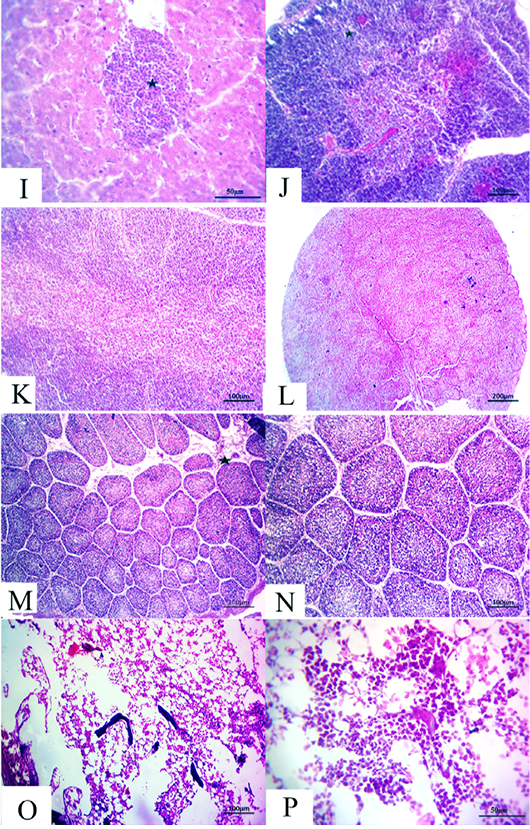
Figure 5: (14 days old): (I):High magnification of the liver showing inflammatory cell aggregations primarily mononuclear cells (star), H&E×200. (J):Representative photomicrograph of the thymus section showing nearly normal histomorphological structures with persistent mild depletion of medulla (star) besides widening of blood vessels, H&E×100. (K):Representative photomicrograph of the thymus section showing nearly normal histomorphological structures with persistent mild depletion, H&E×100. (L):Representative photomicrograph of the spleen section showing marked depletion of white pulpm H&E×100. (M):Representative photomicrograph of the bursa of Fabricius section showing marked interstitial edema (star) with prominent depletion of all follicles, H&E×100. (N):High magnification of the bursa of Fabricius showing the depletion of all follicles, H&E×200. (O):Representative photomicrograph of the bone marrow section showing marked depletion of all hematopoietic contents H&E×100. (P): High magnification of the bone marrow showing marked depletion of all hematopoietic contents H&E×200.
flammatory cell aggregations primarily consisting of mon onuclear cells besides mild congested blood vessels (Fig. 5I). Moreover, the thymus section demonstrated nearly normal histomorphological structures with persistent focal depletion in the cortex in addition to widening of blood vessels (Fig. 5J). A few sections displayed nearly normal histomorphological structures with persistent mild depletion (Fig. 5K). The splenic section revealed markedly depleted white pulp in the majority of the examined cases (Fig. 5L). The bursa of Fabricius section showed prominent depletion of all follicles with marked interstitial edema (Fig. 5M and N). The bone marrow section exhibited marked depletion of all the hematopoietic contents with persistent prominent lymphocytic series (Fig.5O and P).
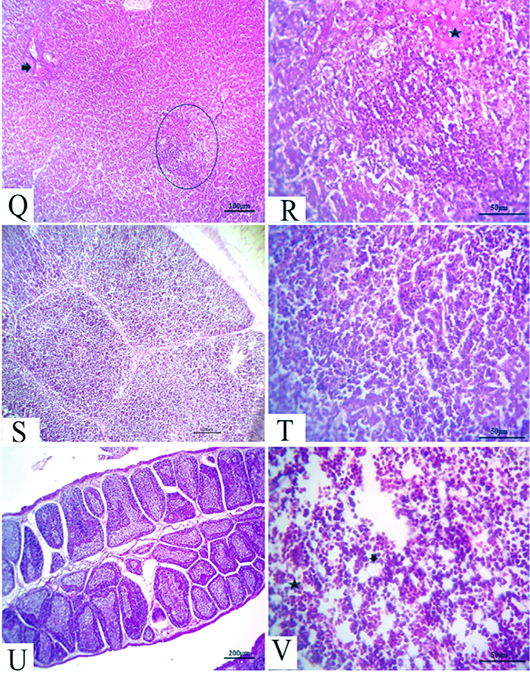
Figure 6: (21 days old): (Q):Representative photomicrograph of the liver section showing nearly normal hepatic structures with persistent mild congestion of blood vessels (arrow) besides persistent inflammatory cell aggregations, H&E×100. (R):High magnification of the liver showing lymphocytic aggregations with edema (star), H&E×200. (S):Representative photomicrograph of the thymus section showing nearly normal thymus structures, H&E×100. (T):Representative photomicrograph of the splenic section showing nearly normal splenic structures, H&E×100. (U):Representative photomicrograph of the bursa of Fabricius section showing nearly normal histomorphological structures H&E×100. (V):High magnification of the bone marrow showing all hematopoietic series (star) with limited fat globules (arrow), H&E×200.
After 21 days, the CAV-infected birds showed complete nearly normal hepatic architectures, with a few birds still suffering from mild congested blood vessels in addition to persistent inflammatory cells aggregations (Fig.6Q and R). Moreover, nearly normal thymus, bursa of Fabricius, and splenic structures were observed (Fig.6S, T and U). Meanwhile, the bone marrow section exhibited nearly normal structures, including all the hematopoietic series with limited fat globules (Fig. 6V).
Immunofluorecent findings: Fluorescent green-apple-colored CAV viral particles were observed inside the nuclei of hematopoeitic cells of thymus (Fig. 7A and B), bone marrow (Fig. 7C), and spleen (Fig. 7D).
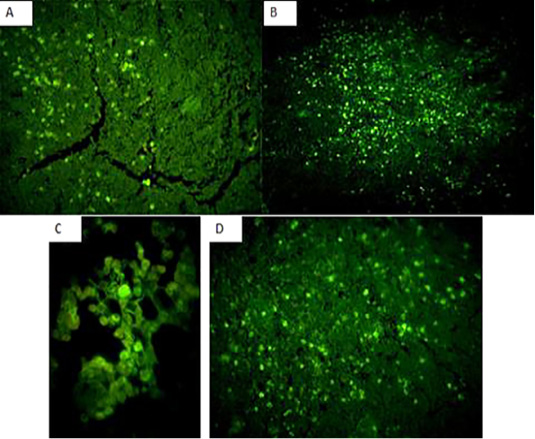
Figure 7: Fluorescent green-apple-colored CAV viral particles were observed inside the nuclei of hematopoeitic cells of the infected group,×200. (A and B): thymus,×200, (C): bone marrow,×400, and (D) spleen,×200.
Discussion
CAV is a disease affecting young chickens characterized by generalized lymphoid atrophy resulting in immune suppression and severe destruction of erythrocytic and granulocytic series of the bone marrow cells producing aplastic anemia (Taniguchi et al., 1983). The mortality rate is generally between 10% and 20%, but it may reach 60%, especially in complicated cases (Gelderblom et al., 1989).
PCR is considered as an assay of choice for the detection of CAV-DNA in chicken tissues. It has been confirmed to be specific and definitely more sensitive than cell culture isolation of the virus (Miller et al., 2001). In the present study, PCR analysis of the DNA sample extracted from the thymus, spleen, liver, bone marrow and bursa of Fabricius of the diseased birds revealed clinical signs and lesions suggestive of CAV-yielded positive reactions with production of specific amplicon at (583 bp), which were consistent with the results of Hegazy et al. (2010).
Inoculation of 1-day-old chicks free of maternal antibodies can be used to isolate and propagate CAV in instances where the clinical syndrome suggests that CAV is present, but in vitro virus isolation and/or PCR assays are negative. Bioassay through intramuscular inoculation of susceptible 1-day-old chicks is the most sensitive method available for the primary isolation of CAV (Schat, 2003). This approach can be used if CAV is suspected, but the virus is not isolated in cell culture. Between 14 and 21 days P.I., hematocrit values below 27% are considered to be indicative of the presence of CAV (Rosenberger and Cloud, 1989a; Lucio et al., 1990). It is important to confirm whether CAV is present by PCR or immune histochemistry (Schat, 2003). Therefore, we confirmed our diagnosis by intramuscular inoculation of susceptible 1-day-old chicks and detected the subsequent results and then via immunohistochemical changes.
The clinical signs and postmortem lesions observed in our study were in accordance with those reported by Yuasa et al. (1979) and Ali, (2001). In fact, yellowish changes in the bone marrow and thymic atrophy may be indicative of chicken anemia virus infection in addition to other methods of diagnosis (Ramadan et al., 1998).
The hematological picture of the infected group revealed aplastic normocytic normochromic anemia represented by severe decrease in PCV, RBC count and hemoglobin content with normal MCV and MCHC, which may be due to the direct effect of the virus on the bone marrow leading to aplasia and destructive effect on the erythrocytic and granulocytic series of the bone marrow cells (Taniguchi et al., 1982), which in turn affect erythropoiesis and myelopoiesis (Schat, 2003; Dhama et al., 2008). The hematocrit values were markedly reduced (17%–22%), which is characteristic of CAV disease, and provided a rapid recognition of the suspected infected birds, which was consistent with the result of Hegazy et al. (2014). Anemia appeared at 14 days post infection, as previously reported by Wani et al. (2014) who showed that the PCV value of the infected chicks was significantly low (18.22±2.22) compared to that in the control group (34.12±4.72) at 14 days post infection. Furthermore, VonBulow and Schat, (1997) also mentioned that chicks experimentally infected at 1 day of age developed lesions 8 days P.I. and anemia at 12–16 days P.I.
In this study, marked hypocellular bone marrow was observed represented by depletion of all blood cells and their precursors and their replacement with adipose tissue which slightly improved at 21 days old. This may be due to the destructive effect of the virus on bone marrow cells, as previously reported by Rosenberger and Cloud, (1998) who demonstrated that lesions of uncomplicated CAV in young chickens typically consist of bone marrow aplasia. Similarly, Smyth et al. (1993) also reported that the primary cells targeted during the pathogenesis of CAV infection include hematopoietic precursor and thymic precursor cells in the bone marrow and thymus cortex, respectively. Hemocytoblasts are specialized bone marrow cells from which the erythroid and myeloid series are derived. Hemocytoblasts in the bone marrow are among the first cells in which CAV antigen is detected at around 3 ± 4 days after infection at 1 day old (Noteborn, 2004). The hypoplasia observed in this study or the aplasia involving the hematopoietic cell lineages was in agreement with previous reports (Smyth et al., 1993; Adair, 2000; Dhama et al., 2002), which described that the hemocytoblasts in the bone marrow and the precursor lymphocytes in the thymus are the major targets for CAV. The destruction of hemocytoblasts results in severe decreases in erythroid and myeloid cells, which ultimately leads to the anemia characteristic of the disease (Drén et al., 2000). These destructive changes are observed in the blood leading to decreased hematocrit levels and number of circulating leukocytes from 8 days post infection (Adair, 2000).
Hypoproteinemia, hypoalbuminemia, hypoglobulinemia, and a decrease in the A/G ratio in the infected group may be due to anorexia, decreased feed intake, kidney and liver damage observed in the histopathological examination, in addition to the immunosuppression that occurred in the infected birds, which was consistent with the observations of (Gelderblom et al., 1989) and (Hegazy et al., 2010) who reported that the immunosuppressive effect of the virus may be attributed directly to the destructive effect of the virus on the hematopoietic and lymphopoietic tissues.
There were significant increases in the levels of the liver enzymes ALT and ALP and a significant decrease in triglyceride (TG) levels due to liver and kidney damage ( McNulty, 1991; Dhama et al., 2008) as the liver is responsible for their metabolism and ALP. Our results are also consistent with those of (Krishan et al., 2015). The histopathological lesions further confirmed this result. Furthermore, the anorexia affecting the birds decreases the levels of blood proteins and TG. There were significant decreases in Ca and inorganic phosphorus levels due to anorexia and a decrease in the feed intake related to the hypoalbuminemia.
In this study, histopathological examination of the experimentally infected broiler chickens revealed mild, moderate to severe changes in the lymphoid tissues (thymus, bursa, spleen, liver, and bone marrow). These changes were in the form of focal to diffuse depletion of lymphocytes and hematopoietic cells. There were marked degeneration and necrosis of lymphocytes within the lymphoid tissues. Our results were consistent with those of Sakr and Talaat, (1991) who observed marked depletion of lymphocytes in the thymus and the bursa of Fabricious addition to severe hypoplasia in hematopiotic cells in the bone marrow. Furthermore, (Hussein et al., 2002) reported some bursal changes with a degree of atrophy in lymphoid follicles with scattered necrotic foci. Similar lesions of CAV were observed by (Dhama et al., 2002, Kuscu and Gurel, 2008) who detected the virus in the bone marrow, thymus, and spleen at 6–7 days post infection. In addition, (Smyth et al., 1993) indicated that CAV replicates in thymic lymphoblasts and reticular cells with lymphocytic depletion in the thymic cortex and hypoplasia of the bone marrow.
Hegazy et al. (2010) reported that the bursa of Fabricius of CAV-infected birds displayed moderate depletion of lymphoid follicles. Marked depletion of lymphocytes was observed in the thymus and the bursa of Fabricius in addition to hypoplasia in hematopoietic cells in the bone marrow. Similarly, (Aly et al., 2000) mentioned that the parenchymatous organs of experimentally infected chickens exhibited infiltration of mononuclear cells with focal necrosis of some hepatic cells and parenchymal edema and mild congestion with thickening in the blood vessel wall.
The immunohistochemical findings revealed intranuclear fluorescent green-apple-colored antigenic particles specific for CAV in the thymus, bone marrow and spleen of the infected group, this was in accordance with (Brown et al., 2000; Wani, et al., 2014) and this was confirmatory diagnosis of CAV infection.
Conclusion
In the present study, we identified that CAV has destructive effects on thymus, spleen, and bone marrow, thereby causing severe aplastic anemia, marked decrease in PCV, and immunosuppression. It also affects liver, causes impairment in liver functions, and increases the activity of its enzymes. We confirmed the diagnosis of CAV infection by reinfection of one day old SPF chicks and by observing the clinical signs which were stunting, growth retardation, and droopy appearance, anorexia, depression, generalized weakness, pale comb and wattles. PM lesions were atrophied thymus glands, subcutaneous hemorrhages, yellow fatty bone marrow, and atrophied bursa of Fabricius. Anemia and severe decrease in PCV to <22% were also observed. In addition to the detection of fluorescent green-apple-colored CAV viral particles in the target organs.
Acknowledgments
Great thanks to Pathology and Virology units in Poultry diseases department, Animal Health Research Institute, Dokki, Cairo, with special thanks to Prof. Dr. Lila A Tantawy for their help in the immunofluorecent studies.
Conflict of interest
The authors declare that there is no conflict of interest in the current work.
Ethics statement
The protocol was conducted according to the local animal welfare regulation on experiments with chickens and similar species and the Ethics Committee for Animal Studies at the Faculty of Veterinary Medicine, Zagazig University, Egypt and Animal Health Research Institute, Dokki, Cairo.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
authors contribution
All authors sharing in the project design and performed all investigations, and data analysis equally. They are all sharing in the preparation of the manuscript (writing and revision) and approved the final version of it before publication.
References





