Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Endoparasites and Ectoparasites of Captive Peafowl Farm
Saroj Kumar Yadav1*, Sudeb Sarkar2, Suma Sarkar3, Amam Zonaed Siddiki4
1PhD fellow (Surgery), Department of Medicine and Surgery, Chittagong Veterinary and Animal Sciences University (CVASU), Bangladesh; 2Veterinary Surgeon and Upazilla Livestock Officer, Nazirpur, Pirojpur, Bangladesh; 3Veterinary Surgeon and Upazilla Livestock Officer under Department of Livestock Services, Bangladesh; 4Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong-4202, Bangladesh.
Abstract | Ectoparasite and endoparasites in captive peacock are widely distributed in the world. As there appeared to be fewer data available published in scientific journals on parasitic infection of a captive peacock in Bangladesh and considering the potential threat of captive peafowl for animal and public health, the present study was carried out by using fecal samples collected from peafowl farm of Banskhali in Chittagong city of Bangladesh. A total of 50 captive peafowls out of 50 peafowls, 10 (41.67%) were infected with both coccidiosis and ascariasis. Only Coccidia out of 50, 30 (60%) was infected and only Ascariasis out of 50, 24(48%) were infected. Ectoparasites (Amyrsidea minutes) out of 50, 50 (100%) were infected. The female was more likely infected compared to the male but was statistically insignificant (p>0.05). Floatation method has significantly higher accuracy to diagnose coccidiosis in peafowl than the sedimentation method, whereas sedimentation method has 100% accuracy to detect ascariasis in positive samples of 50.
Keywords | Prevalence, Endoparasite, Ectoparasite, Captive peacock
Received | March 23, 2019; Accepted | December 08, 2020; Published | January 15, 2021
*Correspondence | Saroj Kumar Yadav, PhD fellow (Surgery), Department of Medicine and Surgery, Chittagong Veterinary and Animal Sciences University (CVASU), Bangladesh; Email: Shirfraaz@gmail.com
Citation | Yadav SK, Sarkar S, Sarkar S, Siddiki AZ (2021). Prevalence of endoparasites and ectoparasites of captive peafowl farm. Adv. Anim. Vet. Sci. 9(3): 442-445.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.442.445
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Yadav et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Peafowl is a member of the Galliformes bird of the family Phasianidae. According to origin, they are categorized into two groups, the Asiatic species, commonly known as Indian or blue peafowl (Pavo cristatus) of India and Sri Lanka and the green peafowl (Pavo muticus) of Burma, Indochina, and Java, whereas the second group is the Congo peafowl (Afropavo congensis) of African species (Sun et al., 2014). Moreover, the Indian blue peafowl has a strain of white peafowl. The male peafowl is called Peacock and a female is known as a peahen. Historically, till now peafowl in the jungle are distributed in India, Pakistan, Sri Lanka, Nepal, and Myanmar. But nowadays, have been maintained in captivity across the world (Khursheed et al., 2014). In general, it is not surprising that, like other poultry species, peafowl’s are parasitized by certain endoparasites such as nematodes and protozoa. Though less is known about the parasitic diseases of peafowl, it accepts the fact that most diseases have resembled the ones documented in turkeys (Titilincu et al., 2009). Among parasitic diseases caused by protozoa, coccidiosis is common and causes the most rigorous health and economic problems (El-Shahawy, 2010). The effect of gastrointestinal endoparasitism may range from simply a behavioral deviation to death. The common symptoms of gastrointestinal parasitic diseases include poor body condition, loss of appetite, greenish or reddish droppings, huddling together, heads drawn in and ruffled feathers (Qamar et al., 2013). Owing to this, the affected bird’s losses its value for exhibition and the aesthetic purpose of fancy rearing. In a previous study, the prevalence was found over 50% of both coccidial and nematode infections from peacock to a household farm in Romania (Titilincu et al., 2009). Although, peafowl farming is not a new business idea in a global perspective, but is a new concept for many corners of the world like Bangladesh. Yet, fancy peafowl rarer and the researchers do not have plenty of information regarding gastrointestinal parasitic infection of peafowl despite a high risk of infection. Therefore, the objective of the study was to investigate the prevalence of ascariasis and coccidiosis in household captive peafowl and to assess the ability of coproscopic diagnostic methods to diagnose these diseases in Chittagong, Bangladesh.
MATERIALS AND METHODS
Sample collection
The study was conducted a fancy peafowl farm located in Banshkhali Upazila of Chittagong, Bangladesh. The eggs of the studied peafowl were collected from England and incubated and hatched to chicks in Bangladesh. The peafowl is reared in captivity enclosed by a net, but have sufficient roaming space within it. Samples were collected from all 50 peafowls from the farm directly from the rectum using cotton swab during August 2016. Samples were placed in 10% formalin containing sterile vials labeled with unique identity numbers, transferred to the laboratory and stored at 4°C until being evaluated and ectoparasites were collected by Visual examination in which the ectoparasites were collected from host birds at Banskhali farm. The legs of host birds immobilized with a strip of surgical tape (Lee and Clayton, 1995) (Figure 4) and then with the help of both hands full body regions examined. Then all collected parasites were preserved in 70% alcohol and 1 drop of glycerin added to prevent evaporation. The preserved samples were brought to the laboratory at Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh.
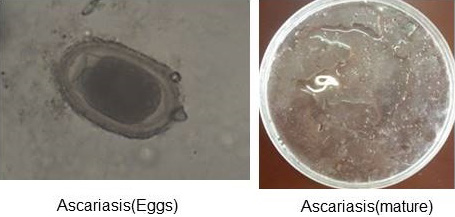
Figure 1: Ascariasis (Eggs and mature).
Laboratory examination of samples
Two different types of qualitative analyses, namely floatation and sedimentation technique were followed to detect the eggs in the fecal materials. The direct smear method for fecal examination was also performed, as described by Hossain et al. (2011). At least three smears were prepared for each sample and eggs were identified on the basis of their morphological features (Soulsby, 1986). (Figure 1 and Figure 2) In addition, the Modified McMaster Counting technique as described by Soulsby (1986) was performed to determine the parasitic load (egg per gram). And The preserved ectoparasite samples were brought to the laboratory of Department of veterinary and animal sciences. They were identified by the method of (Durden, 2002) on the basis of their morphological characters, (Figure 3) following the identification keys (Holland, 1985; Price et al., 2003; Verma, 1993).
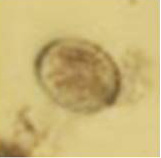
Figure 2: Oocyst of Coccidia.
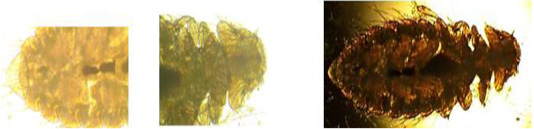
Figure 3: Amyrsidea minutes.

Figure 4: Method of collection ectoparasites.
Data analysis
Field and laboratory data were entered into the Microsoft Excel Program 2007. Data were sorted and checked the integrity before exporting to STATA 13 (Stata Corp, 4905, Lakeway Drive, College Station, TX 77845, USA) for epidemiological analysis. Descriptive analysis was performed on qualitative and quantitative results of endoparasite. The results were presented as percentage, mean and 95% confidence interval. The set level of significance was ≤0.05. A value of p ≤0.05 was considered significant in all statistical tests at a confidence interval of 95%.
RESULTs
Overall qualitative prevalence of coccidiosis and ascariasis are displayed in Table 1. Out of 50 peafowls, 10 (41.67%) were infected with both coccidiosis and ascariasis. The female animals were more likely to infect in compared to the males, but was statistically insignificant (p>0.05) are displayed in Table 2. Floatation method has significantly higher accuracy to diagnose coccidiosis in peafowl than the sedimentation method, whereas sedimentation method has 100% accuracy to detect ascariasis in positive samples (Table 3) and ectoparasite 100% out of 50 (Amyrsidea minutes).
Table 1: Qualitative and quantitative prevalence.
| Overall | Egg/oocyst per gram value | ||||
| Endoparasite | Observation N | No of positive n (%) | Mean | Std. dev. | CI |
| Coccidia | 50 | 30 (60) | 376 | 584.00 | 0-2500 |
| Ascaridia | 50 | 24 (48) | 164 | 427.08 | 0-2100 |
| Parameters | Observation | Coccidia | Ascaridia | Coccidiosis + Ascariasis | |
| Male | 18 | 12 (66.67) | 10 (55.56) | 6 | 10 (41.67) |
| Female | 32 | 18 (56.25) | 14 (43.75) | 4 | |
| P value | 0.305 | 0.452 | |||
Table 3: Comparison of diagnostic methods to detect the ability of positive result detection.
| Parameters | No of positive | Floatation method | Sedimentation method | P value |
| Coccidia | 30 | 30 (100) | 10 (33.33) | 0.000 |
| Ascaridia | 24 | 10 (41.67) | 24 (100) | 0.001 |
DISCUSSION
Parasitic infestation is one of the major problems causing mortality in wild animals in captive form (Rao and Acharjto, 1984). Zoo birds under captivity suspected to anemia, reduce growth, weight loss, illness and skin damage due to ectoparasites. Heavy infestations sometimes cause the death of host (Arnall and Keymer, 1975) The droppings of peafowl were blackish-green in color, semisolid with mild streaks of urates. The fecal samples revealed a wide spectrum of nematodes and coccidian oocysts. Freshly egested fecal samples (n=50) were collected from peacocks (Pavo cristatus) from August 2016 at Banshkhali Upazila of Chittagong, Bangladesh. These fecal samples were examined concerning the presence of oocysts of Eimeria and Eggs and live of Nematode (ascariasis). Out of 50 fecal samples examined peafowl, 10 (41.67%) were infected with both coccidiosis and ascariasis. In this study one species of lice found. The lice in peafowl also noticed (Green and Plama, 1991) but the species of lice different due to a condition of the host environment.
Sex-wise prevalence of coccidiosis and ascariasis: Out of 50 samples examined in August 2016, 18 samples were of male and 32 samples were of a female. Out of 18 male samples, 12 Coccidia and 10 Ascariasis were found positive. The percentage prevalence of disease in males was 66.67% and 55.56 %. Out of 32 female samples, 18 Coccidia and 14 Ascariasis were found positive and the percentage prevalence of disease in females was 56.25% and 43.75%. Both Coccidia and Ascariasis out of 18 males 6 are positive and out of 32 Female 4 are positive so p-value is 10(41.67) (Table 2). In a study carried out by Titilincu et al. (2009), it was found that besides a few countable species of nematodes affecting peacocks, but eight species of coccidia. Coccidiosis is known to cause serious mortality in galliform birds in captivity (Rommel, 2000) but in the current study, also found coccidian more than Nematode. Interestingly, Eimerian parasites of birds are generally considered to highly host specific not only under natural conditions (Hiepe and Jungmann, 1983) but also in farmed birds (Rommel, 2000). Therefore, host systematic and geographic origin are commonly used criteria in their taxonomy. Likewise, almost nothing is known on the seasonality and biology of coccidian infections in galliform captive birds. However, knowing how severe disease and high mortality can be caused in galliform birds in captivity (Rommel, 2000). More detailed parasitological studies are needed and future research on game-bird population dynamics should not neglect protozoan infections, particularly the ones caused by coccidian parasites, which are of great importance for species conservation.
Ectoparasite prevalence
Ectoparasites were collected by Visual examination in which the ectoparasites were collected from host birds at Banskhali farm. The legs of host birds immobilized with a strip of surgical tape (Lee and Clayton, 1995). 100% number are affected by ectoparasite (Amyrsidea minute). All juveniles probably receive their lice from their mothers during the extended periods of close contact involved in brooding. However, it is possible that lice transfer from the male to the female during copulation (Durden, 1983).
ACKNOWLEDGMENTS
We express our sincere thanks to the Department of pathology and parasitology, Chittagong Veterinary and Animal Sciences University, Chittagong-4202, Bangladesh
Author’s Contribution
All authors have equal contribution.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





