Advances in Animal and Veterinary Sciences
Research Article
Effect of Pueraria Flavonoid Supplementation against LPS Infusion in Sheep
Walaa I. Mohamaden1,2,3, Ibrahim M. Hegab4, Chen Hui1,2, Shi Shang-li1,2*
1College of Grassland Science, Gansu Agricultural University, Lanzhou 730070, China; 2Key Laboratory of Grassland Ecosystem, Ministry of Education, Sino-U.S. Centers for Grazing Land, Lanzhou 730070, China; 3Department of Animal Medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt; 4Department of Hygiene, Zoonosis and Animal Behavior and Management Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Walaa I. Mohamaden, Ibrahim M. Hegab and Chen Hui contributed equally to this study.
Abstract | Using natural bioactive compounds as dietary supplements for animals’ diet seems to be a great strategy to improve the animals’ immunity and resistance against diseases. Flavonoids are well-known of their beneficial effects on health, growth, and performance. Our experiment was conducted to determine the anti-inflammatory and antioxidant effects of puerariae flavone dietary supplementation on sheep in different doses after exposure to lipopolysaccharides (LPS). Twelve small tailed Han ruminally-cannulated rams were randomly assigned to three groups; control group; fed with a basal diet, and 50FLV and 100FLV groups which were offered the same diet and further administered with 50 mg or 100 mg of puerariae flavonoid/ kg body weight through rumen cannula for two weeks, respectively two times daily. At the end of the experiment, all animals were infused with LPS. Animals were monitored before and after infusion, clinical body parameters were recorded, and plasma samples were collected for analysis. Body temperature was significantly increased in all groups. There were significant increases in the IL-6, iNOS and COX-2 plasma levels of 50FLV group. Malnodialdhyde (MDA) levels tended to increase in control group compared to the other groups, meanwhile Glutathione (GSH) levels were significantly increased in 100FLV group. We concluded that adding 50 mg of puerarine /kg to sheep diet would enhance the inflammatory response against infection.
Keywords | GSH, Inflammatory markers, Lipopolysaccharides, Puerariae flavonoids, Sheep
Received | June 18, 2020; Accepted | October 23, 2020; Published | January 01, 2021
*Correspondence | Shi Shang Li, College of Grassland Science, Gansu Agricultural University, Lanzhou 730070, China; Email: shishl@gsau.edu.cn
Citation | Mohamaden WI, Hegab IM, Hui C, Shang-li S (2021). Effect of pueraria flavonoid supplementation against lps infusion in sheep. Adv. Anim. Vet. Sci. 9(2): 295-300.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.295.300
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mohamaden et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Boosting the herd’s immunity is a very important goal in animal production industry. The best way to accomplish a high immunity level is to feed animals on a well-balanced diet supplemented with appropriate feed additives. Indeed, some feed additives provide a great effect on enhancing animal resistance against diseases and reducing illness signs (Zhan et al., 2017). Livestock animals are fed on grasses which contain different levels of polyphenolic compounds which are responsible for the flower’s pigmentation, plant growth and development, and blossom formation (Dudek et al., 2016). At the same time, they play an important protective role in the plant–microbe and plant animal interactions (Winkel, 2001), beside its effect on rumen digestibility of nutrients (Mohamaden et al., 2020).
Flavonoids are naturally occurring polyphenols, and inclusion of flavonoids into the diet can improve liver antioxidant capacity in ruminants (Gladine et al., 2007). Animal’s health and immunity are also improved corresponding to better lactation performance, milk yield and animal growth (Hu and Zhang, 2009; Metwally et al., 2013). Furthermore, flavonoids act as anti- inflammatory agents by inhibiting the production of prostaglandins and limit the generation of some other pro-inflammatory cytokines (Manthey, 2000; Tunon et al., 2009).
Puerarin (C21H20O9) is a natural flavonoid compound extracted from the traditional Chinese herb Radix puerariae, which has been used for the treatment of several diseases in the traditional Chinese medicine (Li et al., 2013; Chen et al., 2013; Peng et al., 2013). Puerarin possesses various pharmacological properties, including antioxidant, anti-inflammatory, anti-apoptosis (Zhang et al., 2018). Its therapeutic effect is completely satisfactory in several diseases, both in animal and clinical studies (Li et al., 2016; Jiang et al., 2016). Puerariae flavone was recoded to regulate the cytokine balance and inhibit the inflammatory reaction in experimentally injured liver model in rats (Zhou et al., 2018).
Once the lipopolysaccharides (LPS) or bacterial endotoxins enter the circulation, they activate an innate immune reaction and stimulate the development of inflammatory process. Inflammation is a protective response that eliminates noxious stimuli and promotes the regeneration of damaged tissue (Eom et al., 2018). Therefore, we assumed that supplementation of sheep diet with some feed additives which have a positive effect would induce a great influence on the animal’s health and consequently on its response to infection.
The aim of the present study was to assess the effect of puerariae flavonoid through supplementation of sheep on different doses of puerariae flavone and measure the plasma antioxidant levels, in addition to record the inflammatory response before and after LPS infusion.
MATERIALS AND METHODS
Animals and experimental design
Twelve small tail Han ruminally-cannulated rams fed on green fodder, wheat bran, sorghum straw, grains, corn stalk, soybean meal, and a cereal mixture of wheat skin, oats, sorghum, millet, corn and mineral mixture were used in this study. Animals were randomly allocated into three groups (4 rams per group) according to pueraria flavonoid supplementation (extracted from Puerariae Lobota roots with purity of 81.5%- Baoji F.S. biological development Co., LTD. China). The control group [42.65±3.44 Kg body weight (BW)] was not supplemented with flavonoids, while 50FLV (43.70±3.12 Kg BW) and 100FLV (42.65±1.53 Kg BW) groups were provided with 50 or 100mg puerariae flavonoid /kg of body weight administered through rumen cannula respectively. Supplementation of puerariae flavonoid was given twice daily for two successive weeks. All procedures involving animals were according to animal care and welfare guidelines of Gansu Agricultural University Institutional Animal Care and Use Committee.
Infusion
Immune stress was induced by intravenous infusion though jugular catheter with LPS of Escherichia coli 055:B5 (Sigma, Shanghai Sheng Biotechnology Ltd; Co.) at the dose of 0.5μg /kg of body weight, dissolved in saline (0.9%w/v NaCl) at a concentration of 10 mg/L (10μg/mL). Each animal needed about 2-2.6 ml dissolved in 100 ml 0.9% NaCl depending on the body weight. The immune stress was applied at day 15 of the experiment.
Clinical measurements and sampling
All measurements were made immediately before LPS infusion (0h), at 3h post infusion (3hpi), 6hpi, 24hpi and 48hpi. Rectal temperature was measured using a mercury thermometer, heart rate was measured heartbeat/min (HB/min) by auscultation using a stethoscope placed on the thoracic cavity, and respiration rate was recorded following timed observation of the abdominal wall respiratory cycles / min (RC/min). Five ml of blood samples were collected in the same times of clinical assessment and stored in tubes with EDTA then centrifuged 3000g/10 minutes and plasma was collected and stored at -20oC for further analysis.
Inflammatory markers, MDA and GSH
Interleukin-6 (IL-6), inducible nitric oxide synthetase (iNOS), cyclooxygenase 2 (COX-2) and nuclear factor- kB (NFkB) were measured in plasma using goat kits (ShagHai Lengton Bioscience Co. China) following the manufacturer’s directions. Malnodialdhyde (MDA) and glutathione (GSH) were measured in plasma by the colorimetric method using spectrophotometer according to the manufacturer instructions (Suzhou Keming Biotechnology Co. Ltd, China). Each sample was measured in triplicate.
Statistical analysis
SPSS 22.00 software (Armonk, NY: IBM Corp.) was used for all analyses and graphical presentations. Mixed analysis of variance (ANOVA) was used to examine the main effects of time (within-subject factor) and group (between-subject factor) and their interaction on different clinical body parameters (Respiratory rate, body temperature and heart rate) and plasma levels of inflammatory markers and antioxidants. Duncan’s post-hoc test was used where appropriate. Differences were declared significant at P≤ 0.05.
RESULTS AND DISCUSSION
Clinical body parameters
After LPS infusion toxemia signs including dullness, depression, anorexia, rapid labored breathing, tachycardia, increased body temperatures were observed. Monitored clinical manifestations results are presented in Tables1, 2 and Figure 1.
Table 1: Clinical parameters (Mean ± SE) at different hours after intravenous LPS infusion in all experimental groups.
| Group | Time | Respiratory rate | Temperature | Heart rate |
| Control | 0 |
20.00±1.16c |
37.93±0.47b |
90.67±5.81c |
| 3 |
26.67±0.33a |
39.80±0.29a |
153.33±12.02a |
|
| 6 |
25.67±0.33ab |
39.53±0.03a |
130.00±5.77b |
|
| 24 |
22.33±1.45bc |
39.63±0.12a |
115.33±2.91b |
|
| 48 |
24.33±1.45ab |
39.20±0.17a |
120.00±2.31b |
|
| 50 FLV | 0 |
28.00±2.35c |
39.50±0.22b |
111.00±8.43 |
| 3 |
57.75±4.66a |
40.93±0.06a |
119.75±43.61 | |
| 6 |
48.25±4.80ab |
40.40±0.28a |
162.50±24.19 | |
| 24 |
42.50±6.12abc |
39.43±0.22b |
151.25±13.55 | |
| 48 |
34.25±5.39bc |
39.50±0.23b |
144.00±4.69 | |
| 100 FLV | 0 | 30.25±1.44 |
38.45±0.16c |
111.25±5.99 |
| 3 | 39.25±10.46 |
40.78±0.14a |
116.50±18.32 | |
| 6 | 34.00±3.46 |
39.83±0.20b |
136.00±22.33 | |
| 24 | 27.75±4.84 |
38.85±0.38c |
137.25±12.84 | |
| 48 | 31.00±5.92 |
38.88±0.37c |
106.50±10.70 |
Means with different superscripts within the same group at the same column are significantly different at (P ≤ 0.05).
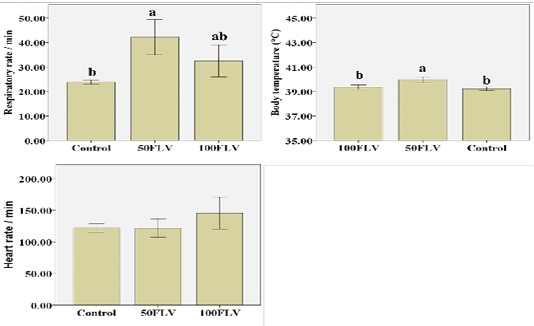
Figure 1: Overall mean of Clinical parameters (Mean ± SE) after intravenous LPS infusion and in all experimental groups. Different letters indicate significant difference among groups (P ≤ 0.05)
Respiratory rate (RR) showed that both time (F4,32= 5.47, P= 0.002, η2 = 0.41) and group (F1,8= 8.19, P= 0.01, η2 = 0.67) significantly influenced the respiratory rate. The greatest RR was recorded at 3 hours post injection (hpi) (42.55 ± 5.48). 50FLV group recorded the highest RR (42.15 ± 3.60) compared to the other groups (Figure 1). Similarly, the body temperature was significantly influenced by time (F4,32= 22.26, P≤ 0.001, η2 = 0.74) and group (F1,8= 13.75, P= 0.003, η2 = 0.77) that the highest body temperature (40.56 ± 0.18) was measured at 3 hpi (Table 2) and the group difference showed that the highest temperature (39.95 ± 0.12) was observed in 50FLV compared to other groups (Figure 1). However, HR was (F4,28= 5.04, P= 0.003, η2 = 0.42) significantly influenced by time that the highest HR (153.33±12.02) was recorded at 3 hpi (Table 2), while there was a non-significant difference (F1,7= 1.23, P= 0.35, η2 = 0.26) in HR amongst groups (Figure 1). Neither RR (F8,32= 1.60, P= 0.16, η2 = 0.29) nor HR (F4,28= 1.18, P= 0.35, η2 = 0.25) were significantly changed by Time × Group interaction, while there was a significant (F8,32= 2.40, P= 0.04, η2 = 0.37) Time × Group interaction effect on the body temperature that the highest temperature was recorded in 50FLV group after 3 hpi (40.93 ± 0.06) compared to the other Time × Group interaction variables.
Inflammatory markers and antioxidants parameters
Biochemical analysis of plasma revealed that there was a significant group effect for iNOS (F2,6= 21.49, P= 0.002, η2 = 0.88), IL-6 (F2,6= 12.15, P= 0.008, η2 = 0.80) and COX-2 (F2,6= 6.11, P= 0.04, η2 = 0.67) concentrations. The highest plasma levels for iNOS, IL-6 and COX-2 (4.37±0.26, 548.86±125.72 and 36.18±1.98, respectively) were observed in the 50FLV group compared to the other treatment groups (Figure 2). A significant group effect (F2,15= 7.29, P=0.01, η2 = 0.49) was also found for the GSH plasma levels, and the highest concentration (0.17±0.07) was recorded in 100 FLV group (Figure 2). However, there were no significant differences neither for NF-kB (F2,6= 1.55, P= 0.29, η2 = 0.34), TNF-η (F2,6= 0.21, P= 0.81, η2 = 0.07) nor MDA (F2,15= 3.46, P=0.06, η2 = 0.32) plasma levels amongst all the groups (Figure 2).
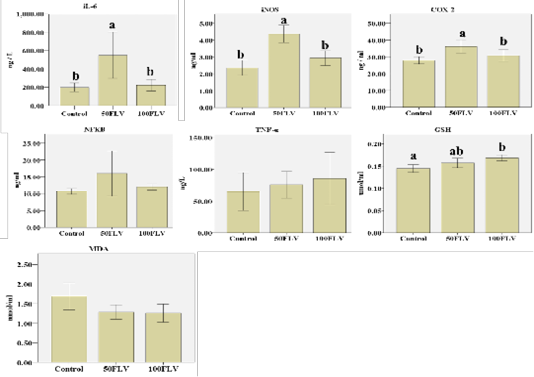
Figure 2: Overall mean of IL-6, I NOS, COX-2, NF-kB, TNF-α, GSH and MDA (Mean ± SE) after intravenous LPS infusion and in all experimental groups. Different letters indicate significant difference among groups (P ≤ 0.05).
Table 2: The overall mean of the clinical parameters (Mean ± SE) at different hours after intravenous LPS infusion in all experimental groups.
| Time | |||||
| Parameters | 0 | 3 | 6 | 24 | 48 |
| Respiratory rate (min) |
26.64±1.63c |
42.55±5.48a |
36.91±3.50ab |
31.64±3.74bc |
30.36±2.94ab |
|
Body temperature (oC) |
38.69±0.25d |
40.56±0.18a |
39.95±0.16b |
39.27±0.18c |
39.19±0.17cd |
| Heart rate (min) |
90.67±5.81c |
153.33±12.02a |
130.00±5.77b |
115.33±2.91b |
120.00±2.31b |
Means carrying different letters within the same parameter are significant are significant at (P≤ 0.05). Respiratory rates (RC/min) and heart rates (HB/min) and temperature oC
Table 3: Plasma concentration levels of IL-6, I NOS, COX-2, NF-kB, TNF-α, GSH and MDA (Mean ±SE) at different hours after intravenous LPS infusion in all experimental groups.
| Group | Time |
IL-6 ng/L |
iNOS ng/mL |
COX-2 ng/mL |
NFKB ng/mL |
TNF-α ng/mL |
GSH µmol/ml |
MDA nmol/ml |
| Control | 0 | 226.22±0.59 | 1.99±0.01 |
27.81±0.30ab |
10.44±0.53 |
30.44±0.99b |
0.14±0.01ab |
2.58±0.55a |
| 3 | 285.08±101.57 | 2.75±0.74 |
29.35±0.85a |
10.60±0.91 |
42.43±6.66b |
0.15±0.00ab |
2.00±0.21ab |
|
| 6 | 173.88±29.92 | 3.10±0.40 |
29.83±0.14a |
9.44±0.21 |
131.83±34.83a |
0.14±0.01ab |
1.02±0.15b |
|
| 24 | 144.24±41.06 | 1.80±0.11 |
22.40±2.05b |
12.00±1.26 |
26.40±2.62b |
0.16±0.01a |
1.25±0.16b |
|
| 48 | 165.43±7.40 | 2.15±0.59 |
30.26±3.44a |
10.92±1.37 |
92.63±46.88ab |
0.13±0.01b |
1.59±0.55ab |
|
| 50 FLV | 0 |
218.34±20.87b |
3.77±0.09b |
34.21±3.64 | 14.14±1.08 | 72.13±11.75 |
0.17±0.01ab |
1.79±0.18a |
| 3 |
445.67±88.26ab |
5.40±0.19a |
32.11±4.67 | 10.66±0.57 | 87.72±8.70 |
0.18±0.01a |
1.54±0.15ab |
|
| 6 |
1119.10±484.18a |
5.26±0.78a |
44.57±5.00 | 28.88±16.72 | 59.26±15.94 |
0.15±0.00bc |
1.02±0.16b |
|
| 24 |
218.38±36.63ab |
3.87±0.17b |
32.81±1.39 | 11.89±0.78 | 107.66±46.36 |
0.15±0.01bc |
0.98±0.28b |
|
| 48 |
742.79±111.51ab |
3.54±0.23b |
37.22±4.89 | 14.41±1.56 | 51.12±15.39 |
0.14±0.01c |
1.09±0.36ab |
|
| 100 FLV | 0 | 191.27±57.37 | 3.36±0.45 | 30.86±4.40 | 12.46±1.47 | 131.54±51.09 |
0.24±0.00a |
1.67±0.34 |
| 3 | 203.34±60.38 | 2.27±0.39 | 27.83±2.53 | 11.98±1.30 | 38.61±2.38 |
0.17±0.01ab |
1.31±0.12 | |
| 6 | 137.07±7.30 | 3.07±0.54 | 28.38±3.90 | 11.18±0.27 | 120.25±56.89 |
0.18±0.01ab |
1.00±0.18 | |
| 24 | 236.20±40.10 | 2.37±0.32 | 32.34±3.75 | 12.74±1.00 | 20.27±2.75 |
0.15±0.01ab |
0.98±0.22 | |
| 48 | 337.52±128.74 | 3.59±0.52 | 34.08±5.94 | 11.84±1.18 | 116.51±59.22 |
0.10±0.02b |
1.33±0.18 |
Means with different superscripts within the same group at the same column are significantly different at (P ≤ 0.05).
Time had a significant influence on iNOS plasma levels toward an increase at 6 hpi (3.81±0.47) (F4,24= 3.27, P= 0.03, η2 = 0.35). Results for the GSH plasma levels also showed a significant time effect (F4,60= 18.02, P<0.001, η2 = 0.55) toward a decrease at 48 hpi (0.12±0.01). MDA plasma levels were significantly influenced by time (F4,60= 6.34, P<0.001, η2 = 0.30) that the highest plasma levels were recorded at 0 h (2.01±0.23). On contrary to the previous findings, time neither significantly influence IL-6 (F4,24= 2.18, P= 0.10, η2 = 0.27), COX-2 (F4,24= 1.31, P= 0.29, η2 = 0.18), TNF-η (F4,24= 2.09, P= 0.11, η2 = 0.26) nor NF-kB (F4,24= 0.70, P= 0.60, η2 = 0.10) plasma levels amongst different time points Table 3.
A significant Time × Group interaction was detected for iNOS (F8,24= 2.52, P= 0.04, η2 = 0.46) that the highest plasma levels were measured at 3 and 6 hpi in the 50FLV group (5.40±0.19 and 5.26±0.78). Also, significant interactions were also recorded for IL-6 (F8,24= 2.71, P= 0.03, η2 = 0.48) and TNF-α (F8,24= 2.88, P= 0.02, η2 = 0.49) plasma levels and Duncan’s post hoc test showed that the highest IL-6 level was recorded at 6 hpi in the 50FLV group (1119.10±484.18), and for the TNF-α at 0 h in the 100FLV group (131.54±51.09) and 6 hpi in the control group (131.83 ± 34.83). Moreover, a significant interaction was also recorded (F8,60= 7.39, P<0.001, η2 = 0.50) for the GSH plasma levels that the highest and lowest GSH plasma levels were measured in 100 FLV group at 0 h and 48 hpi (0.10±0.02 and 0.24±0.00, respectively). Finally, Time × Group interaction was not significant for COX-2 (F8,24= 1.08, P= 0.41, η2 = 0.26), NFKB (F8,24= 1.15, P= 0.37, η2 = 0.28) nor MDA (F8,60= 0.50, P=0.76, η2 = 0.06) plasma levels. However, the highest numerical value for NFKB plasma level was at 6 hpi in the 50FLV group (28.88±16.72). Also, the lowest numerical values for MDA plasma levels were observed in 50FLV and 100FLV groups at 24 hpi (0.98±0.28 and 0.98±0.22, respectively) and in the 100FLV, 50FLV and control groups at 6 hpi (1.00±0.18, 1.02±0.16 and 1.02±0.15, respectively).
The usual therapies for treatment of inflammation or sepsis by administration of chemically synthetic drugs become unacceptable in livestock due to the remaining drug residues and their hazardous impact of on food products that pose a danger to food safety (Byarugaba, 2004). Whereas, it was reported that flavonoids remaining residues in the carcass improve meat shelf-life and microbial safety (North et al., 2019). In the present study, all animals showed significant increases in the body temperature, respiratory and heart rates as a common response to toxemia after LPS infusion. These findings mean that supplementation with flavonoids couldn’t prevent the progress of toxemia under the effect of LPS and the increase in body parameters. These results are consistent with the findings Kanashiro et al. (2008) who reported that quercetin flavones administration didn’t alter the febrile condition induced in rats after administration of LPS with different routes. However, we noticed that the temperature was declined faster in the flavonoid administered groups than in control. Endotoxins are recognized in the circulation by Toll-like receptors (TLRs) of host’s phagocytes and macrophages and bind with it to initiate the inflammatory process, and Pro-inflammatory cytokines are released to regulate the host response to infection (Mohamaden et al., 2019). Flavonoids are known to act as anti-inflammatory agents though suppression of the COX-2, NF-kB and inhibition of the generation of iNOS and so decrease the production of interleukins such as IL-6 and inflammatory mediators as TNF-α (Serrano et al., 1998; Miles et al., 2005; Hämäläinen et al., 2007). The pro-inflammatory markers results revealed that puerariae flavonoid when supplemented to sheep’s diet in different doses induced different effects on inflammatory response to toxemia. In the control group, LPS induced a significant increase in TNF-α plasma levels in the first 6 hpi, while no changes were observed in IL-6, COX-2 levels similar to the results obtained by Eom et al. (2018) when stimulated the peritoneal macrophage cell by LPS. However, 50FLV group showed a significant increase in IL-6, iNOS and COX-2. These results are consistent with those found by Oliviera et al. (2010) who reported that dietary supplementation of calves with polyphenol rich extract enhanced the inflammatory process and increased the synthesis of interferon-γ and interleukin-4, and that indicates the contradiction between the in vitro and in vivo studies. It is important to mention that the inhibitory effect of puerarin of kudzu root extract to IL-6, TNFα and COX-2 is not as intense as that of leaf extract as reported by Eom et al. (2018). In addition, a few flavonoids are categorized as selective COX-2 inhibitors (Levita et al., 2017). The circulating LPS stimulates the production of reactive molecules such as oxygen (O2), hydrogenperoxide (H2O2), nitrogen monoxide (NO), and Malnodialdhyde (MDA) (Dong et al., 2018; Naime et al., 2019). In the present study GSH was significantly increased in 100 FLV group similar to Zhao et al. (2015) who reported that puerarin increased the activity of glutathione peroxidase (GSH-Px) in mice, but after LPS infusion the GSH was decreased because exposure of macrophages to LPS increases the free radicals and the release of Nitric oxide, MDA and decrease the GSH (Dong et al., 2018).
CONCLUSIONS AND RECOMMENDATIONS
Flavonoids are natural polyphenol compounds that can alter the inflammatory responses and as well as the antioxidant activity depending on the doses administered. Our data that supplementation of sheep with 50 mg /kg puerariae flavonoid for 14 days increased the inflammatory response resulted in increased the clinical parameters against LPS infusion, while higher dose provided an antioxidant effect which was declined after LPS infusion. Further studies are needed to determine supplementation of diet with flavonoids would provide a desirable or undesirable effect on animals’ health.
Acknowledgements
The authors would like to acknowledge the Integration and Demonstration of Key Technologies for Ecological Animal Husbandry in the Qinghai-Tibet Plateau (Gannon Community) (No. 201203010).
Authors Contribution
Mohamaden WI and Hegab IM carried out the experiment. Chen H helped and supervise the project. Shi SL. conceived the original idea, supervised the project. All authors contributed the final version of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References





