Advances in Animal and Veterinary Sciences
Research Article
Coexistence of Anti-NP-PPRV, VP7-BTV, and NS-FMDV Antibodies among Non-Vaccinated Domestic Ruminants in Hail Saudi Arabia
Ahmed Zein Elabdeen Mahmoud1, Muaz Magzob Abdellatif2,3*, Yahia Hassan Ali2,4
1Hail Veterinary Laboratory, Ministry of Agriculture. Saudi Arabia; 2Department of Biology (Microbiology), Northern Border University, Saudi Arabia; 3Department of Microbiology, Faculty of Vet Science, University of Nyala, Sudan; 4Department of Virology, Central Veterinary Research Laboratory, Khartoum, Sudan.
Abstract | Peste des Petits ruminants (PPR), Blue tongue (BT), and Foot and mouth disease (FMD) are infectious viruses of livestock. The present work aimed to screen for anti-NP-PPRV, VP7-BTV, and NS-FMDV antibodies among non-vaccinated domestic ruminants (n=841) in Hail. Saudi Arabia. Sera were collected randomly from sheep (n=270), goats (n=270), cattle (n=31), and camels (n=270) of different ages and sex and tested at once by NP-PPR, VP7- BTV, and 3ABC-FMD competitive ELISA. The overall prevalence was 51.8% for PPR, 48.9% for BT, and 17.5% for FMD. Higher positivity for PPR (79.3%), BT (74.1%), and FMD (23.3%) was found in sheep. Co-occurrence of antibodies was 32.9% for PPR/BT and 11.2% for PPR/FMD and BT, PPRV/ BTV and FMDV antibodies was detected in sheep (6.5%), goats (4.4%) and camels (0.2%), PPRV and BTV in sheep (17.4%), goats (15.5%) and camels (0.1%), PPRV and FMDV in goats (0.7%) and cattle (0.2%) and FMDV and BTV was detected in goats (0.5%) and camels (0.1%). Significant correlations between seroprevalence and animal species, PPRV and BTV, PPRV and FMDV, BTV and FMDV were estimated. Novel rapid, sensitive, and specific multiplex immunoassays are essential for the detection of co-infection to improve the management of these devastating viruses.
Keywords | Coexistence, PPRV, FMDV, BTV, Antibodies. Saudi Arabia
Received | August 22, 2020; Accepted | December 03, 2020; Published | January 01, 2021
*Correspondence | Muaz Magzob Abdellatif, Department of Biology (Microbiology), Northern Border University, Saudi Arabia; Email: muazm20@gmail.com
Citation | Mahmoud AZE, Abdellatif MM, Ali YH (2021). Coexistence of anti-NP-PPRV, VP7-BTV, and NS-FMDV antibodies among non-vaccinated domestic ruminants in Hail Saudi Arabia. Adv. Anim. Vet. Sci. 9(2): 289-294.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.289.294
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mahmoud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Peste des Petits Ruminants (PPR), Blue tongue (BT) and Foot and mouth disease (FMD) are among the most important viruses in ruminants. PPRV infects domestic and wild ruminants causing significant economic losses in Africa, Middle East, Asia, and new regions (Banyard et al., 2010, 2014). The virus belongs to morbillivirus genus of the Paramyxoviridae (Zahur et al., 2009), it was reported in Saudi Arabia for the first time in 1990 (EME et al., 1990), later it spread to eastern, central, and northern regions (Adel et al., 2004; Boshra et al., 2015; Mahmoud et al., 2016, 2017). BTV is an arbovirus that is transmitted by certain species of Culicoides biting midges, affecting domestic and wild ruminants, it is one of the 22 serogroups in the Orbivirus genus of the Reoviridae (Mertens et al., 2005). It is characterized by high morbidity and mortality in sheep, while the infection is sub-clinical in some domestic and wild ruminants (Ratinier et al., 2011), it was described in different parts of the country (Hafez et al., 1984; Abu Elzein et al., 1998; Housawi et al., 2004a; Yousef et al., 2012b). FMDV is a member of the Apthovirus of the Picornaviridae, it causes direct production losses and indirect losses due to the implementation of control measures (Knight-Jones and Rushton, 2013). Introduction of subclinical animals acts as a source of new serotypes (Hafez et al., 1994; El-Rahim et al., 2016; Mahmoud and Galbat, 2017).
Co-infection may change the clinical picture, epidemiology and hamper diagnosis and preventive measures against these devastating viruses. The present survey was undertaken to highlight evidence of the coexistence of PPRV, BTV, and FMDV antibodies among domestic ruminants in Hail. Saudi Arabia.
MATERIALS AND METHODS
Sampling
Sera (n=841) were collected randomly from non-vaccinated clinically healthy sheep (n=270), goats (n=270), camels (n=270) and cattle (n=31) of different ages and sexes across 9 villages in Hail, Saudi Arabia during 2018 (Table 1), samples were stored at –20ºC until tested.
Serological examination
Samples were tested at once for anti-NP-PPRV, BTV-VP7, and NS-FMDV antibodies using competitive ELISA as follows:
NP-PPR ELISA
The NP-epitopes based competitive ELISA kit (310rue Louis Pasteur, 34790 Grabels, FRANCE) was used for the detection of PPRV-antibodies according to the manufacturer’s protocol (Libeau et al., 1995). Results were presented as sample-to-negative control ratio (S/N%), S/N=50% were read as positive, >50=60% were doubtful and >60% were negative.
VP7- BTV ELISA
Competitive enzyme immunoassay for the detection of antibodies against BT-VP7 protein was used (IDEXX Blue tongue Competition Ab test, 34090 Montpellier, France) according to the recommended procedure. Samples with S/N ≥80% were considered negative, >70% and ˂ 80% were doubtful and ≤70% were positive.
3ABC-FMD ELISA
The 3ABC-FMD ELISA (IDEXX FMD 3ABC Ab test. IDEXX Laboratories, Inc. Westbrook, Maine 04092, USA) was used for the detection of non-structural Polyprotein (NSP), it was performed according to the manufacturer’s instructions. Samples with percentage values >30% were considered positive, >20% were negative and samples between 20 and 30% were suspicious.
Statistical analysis
Spearman correlation was adopted to estimate the significant correlation of PPRV, FMDV, BTV antibodies and animal species if any (r = 0.7, P-value = 0.01). The analysis was performed using SPSS-22 (Statistical Package for Social Sciences 22).
Table 1: Descriptive analysis of the study area.
| Location | Total | ||||||||||
| Herd NO | Alagfar | Alshamly | Jaba | Hail | Baggaa | Samera | Alhaet | Alshenan | Algazala | ||
| 1 | Count | 18 | 18 | 18 | 23 | 24 | 18 | 18 | 24 | 18 | 179 |
| % within Herd NO | 10.1% | 10.1% | 10.1% | 12.8% | 13.4% | 10.1% | 10.1% | 13.4% | 10.1% | 100.0% | |
| % within Location | 20.0% | 20.0% | 20.0% | 24.2% | 23.1% | 20.0% | 20.0% | 23.5% | 20.0% | 21.3% | |
| % of Total | 2.1% | 2.1% | 2.1% | 2.7% | 2.9% | 2.1% | 2.1% | 2.9% | 2.1% | 21.3% | |
| 2 | Count | 18 | 18 | 18 | 18 | 23 | 18 | 18 | 24 | 18 | 173 |
| % within Herd NO | 10.4% | 10.4% | 10.4% | 10.4% | 13.3% | 10.4% | 10.4% | 13.9% | 10.4% | 100.0% | |
| % within Location | 20.0% | 20.0% | 20.0% | 18.9% | 22.1% | 20.0% | 20.0% | 23.5% | 20.0% | 20.6% | |
| % of Total | 2.1% | 2.1% | 2.1% | 2.1% | 2.7% | 2.1% | 2.1% | 2.9% | 2.1% | 20.6% | |
| 3 | Count | 18 | 18 | 18 | 18 | 21 | 18 | 18 | 18 | 18 | 165 |
| % within Herd NO | 10.9% | 10.9% | 10.9% | 10.9% | 12.7% | 10.9% | 10.9% | 10.9% | 10.9% | 100.0% | |
| % within Location | 20.0% | 20.0% | 20.0% | 18.9% | 20.2% | 20.0% | 20.0% | 17.6% | 20.0% | 19.6% | |
| % of Total | 2.1% | 2.1% | 2.1% | 2.1% | 2.5% | 2.1% | 2.1% | 2.1% | 2.1% | 19.6% | |
| 4 | Count | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 162 |
| % within Herd NO | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 100.0% | |
| % within Location | 20.0% | 20.0% | 20.0% | 18.9% | 17.3% | 20.0% | 20.0% | 17.6% | 20.0% | 19.3% | |
| % of Total | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 19.3% | |
|
5
|
Count | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 162 |
| % within Herd NO | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 11.1% | 100.0% | |
| % within Location | 20.0% | 20.0% | 20.0% | 18.9% | 17.3% | 20.0% | 20.0% | 17.6% | 20.0% | 19.3% | |
| % of Total | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 2.1% | 19.3% | |
|
Total
|
Count | 90.0 | 90.0 | 90.0 | 95.0 | 104.0 | 90.0 | 90.0 | 102.0 | 90.0 | 841.0 |
| % within Herd NO | 10.7% | 10.7% | 10.7% | 11.3% | 12.4% | 10.7% | 10.7% | 12.1% | 10.7% | 100.0% | |
| % within Location | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | |
| % of Total | 10.7% | 10.7% | 10.7% | 11.3% | 12.4% | 10.7% | 10.7% | 12.1% | 10.7% | 100.0% | |
RESULTS AND DISCUSSION
This work aimed to investigate the coexistence of PPRV, BTV, and FMDV antibodies among domestic ruminants in Hail, Saudi Arabia. The prevalence of NP-PPRV-antibodies was 51.8%, sheep exhibited the highest percentage (79.3%) (Figure 1). The incidence of PPRV antibodies was significantly higher in sheep and goats compared to cattle and camels, as a matter of fact, goats and sheep are the natural hosts of the virus (Lefèvre and Diallo, 1990; Abubakar et al., 2008; Salih et al., 2014). The prevalence of PPRV was higher than the reports from Tchad (34%) (Bidjeh et al., 1995), Sudan (51%) (Osman et al., 2009), Algeria (33%) (De Nardi et al., 2012), Tunisia (8%) (Ayari-Fakhfakh et al., 2011) and Saudi Arabia (38.2%) (Mahmoud et al., 2016). In contrast, it was lower than the data from Egypt (63%) (El-Rahim et al., 2010), Libya (59.2%) (Almeshay et al., 2017), Hail (59.9%) (Mahmoud et al., 2017) and Riyadh (64%) (Mahmoud and Galbat, 2017). Discrepancy may be attributed to sample size, diagnostic tests, levels of immunity, and management (Waret-Szkuta et al., 2008). Diverse occurrence of PPRV antibodies in cattle have been published in Kazakhstan (9%) (Lundervold et al., 2004), Pakistan (41.86%) (Khan et al., 2008), India (4.58%) (Balamurugan et al., 2014) and Sudan (25.8%) (Intisar et al., 2017), a higher prevalence may be due to the virulence of the strains involved (Khan et al., 2008; Balamurugan et al., 2012). Natural transmission of PPRV from sheep and goats to cattle has been confirmed without any clinical signs but cattle were seroconverted (Sen et al., 2014). Literature reported the absence of PPRV antibodies in dromedary camels in the Canary Islands and the KSA (Mahmoud et al., 2016, 2017). However, exposure of 2.97% dromedary camels from eastern and south regions of the country to PPR was published by Hemida and Al-Ghadeer (2019). Low percentage may be due to the fatal picture of PPR or to the nature of camel sera (Intisar et al., 2017; Omani et al., 2019), Since, unique classes and antibody binding of camel immunoglobulins was described by Li et al. (2012). Daley et al. (2010) reported divergences in the regulation and function among camelid heavy-chain isotypes on testing immunized alpacas for West Nile virus antibodies. Furthermore, inconsistency of the results obtained by ELISA and virus neutralization in testing camel sera for camel pox virus antibodies was described by Mentaberre et al. (2013), may indicate the need of standardization of ELISA when screening camel sera.
The incidence of VP-BTV-antibodies was 48.9%, a higher rate (74.1%) was found in sheep (Figure 1). The prevalence of BTV antibodies was comparable to the data reported by Sharma et al. (2016). In contrast, it was higher than results found in Ethiopia (46.67%) (Woldemeskel et al., 2000), India (2.63, 29.5 to 45.7%) (Lundervold et al., 2004; Sreenivasulu et al., 2004; Ravishankar et al., 2005), Turkey among small ruminants (29.5%), cattle (18.9%), Albania among small ruminants (4.4%) (Di Ventura et al., 2004), Ethiopia among sheep (29.5-34.93%) (Darsema, 2009; Di Ventura et al., 2004), Iraq (39.47%) (Shlash et al., 2012), Kazakhstan among small ruminants (28.6%) (Bitew et al., 2013), Iran (34.9%) (Khezri and Azimi, 2013) and in Algerian goats (13.7%) and sheep (5.70%) (Kardjadj et al., 2016). In KSA BTV was detected in sheep (54.1%), goats (53.3%), cattle (44.8%) (Yousef et al., 2012b) and camels (1.5-25.7%) (Al-Afaleq et al., 2007; Yousef et al., 2012b).
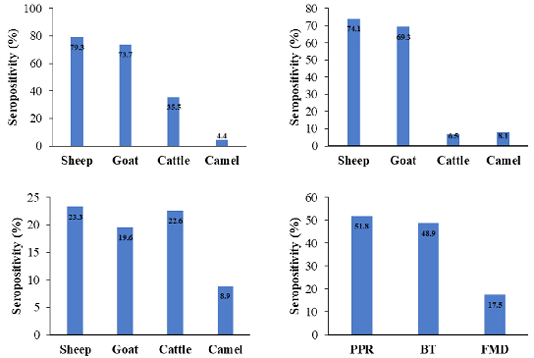
Figure 1: Seroprevalence of viral antibodies among different species as detected by NP- ELISA for PPRV (up left), VP7- BTV ELISA for BTV (up right), 3ABC-FMD ELISA for FMDV (bottom left) and the overall all seroprevalence of antibodies among domestic animals (bottom right).
The prevalence of NS-FMDV-antibodies was 17.5%, sheep presented the highest positivity (23.3%), compared to goats (19.6%), cattle (22.6%), and camels (8.89%) (Figure 1). Infections among sheep are mild or inapparent and often have been considered as disseminators of the virus (Barnett and Cox, 1999; Mohanty et al., 2015). Frequency of 20.1 to 51.5% among cattle (El-Rahim et al., 2016; Lyons et al., 2017), 28.3% in sheep (El-Rahim et al., 2016) was documented in Saudi Arabia during importation. Introduction of animals from enzootic countries might act as a potential source of new serotypes, asymptomatic animals may actively excrete the virus (Hafez et al., 1994). Variation in the prevalence may be due to the virus strains, ecological and geographical factors, serological technique, and the control measures adopted.
Coexistence of PPRV/BTV and FMDV antibodies was confirmed in sheep (6.5%), goats (4.4%) and camels (0.2%), anti-PPRV and BTV antibodies was confirmed in sheep (17.4%), goats (15.5%) and camels (0.1%), antibodies against PPRV and FMDV was detected in goats (0.7%) and cattle (0.2%) and co-occurrence of FMDV and BTV antibodies was proved in goats (0.5%) and camels (0.1%) (Figure 2). A significant correlation between the prevalence of the antibodies and animal species, while a strong positive correlation between PPRV and BTV antibodies was estimated (0.716), a weak correlation between PPRV and FMDV (0.178), BTV and FMDV (0.179) antibodies was observed (Table 2). Data on PPR-FMD infection among small ruminants in Hail and Riyadh, Saudi Arabia were reported (Mahmoud et al., 2017; Mahmoud and Galbat, 2017). Simultaneous infection of PPRV and BTV was described in goats (Malik et al., 2011; Mondal et al., 2009; Saeed et al., 2015). Concurrent infection may be a consequence of immunosuppression induced by PPR as demonstrated by leukopenia, lymphopenia, and reduced immune response (Jagtap et al., 2012).

Figure 2: Coexistence of antibodies against PPRV, FMDV and BTV among sheep, goats, cattle and camels (left) an the overall percentage among domestic ruminants in Hail. Saudi Arabia as detected by Anti-NP ELISA, 3ABC-FMD ELISA and VP7- BTV ELISA.
Table 2: Correlation between the species and prevalence of PPRV, FMDV and BTV antibodies.
| Spp | PPRV | FMDV | BTV | ||
| Spp | Correlation Coefficient | 1.000 |
-.618** |
-.153** |
-.591** |
| Sig. (2-tailed) | .000 | .000 | .000 | .000 | |
| PPRV | Correlation Coefficient |
-.618** |
1.000 |
.178** |
.716** |
| Sig. (2-tailed) | .000 | . | .000 | .000 | |
| FMDV | Correlation Coefficient |
-.153** |
.178** |
1.000 |
.179** |
| Sig. (2-tailed) | .000 | .000 | . | .000 | |
| BTV | Correlation Coefficient |
-.591** |
.716** |
.179** |
1.000 |
| Sig. (2-tailed) | .000 | .000 | .000 | . |
**: Correlation is significant at the 0.01 level (2-tailed).
CONCLUSION
The results provided evidence of concurrent PPRV, BTV, and FMDV infections among farm animals in Hail, Saudi Arabia. Attention should be paid to explore the effects of virus interaction on the pathogenesis, clinical and/or epidemiological features that may hinder the diagnosis and execution of prevention and control policies. Development of rapid, sensitive, and specific multiplex immunoassays is necessary to improve the management against these infectious viruses.
Author’s Contribution
Mahmoud AZE participated in the design of the study, collect samples and perform ELISA tests. Abdellatif MM design the study, participated in the lab work, analysed the results and wrote the manuscript. Ali YH participated in the design of the study, participated in the lab work and revised the manuscript.
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
REFERENCES





