Advances in Animal and Veterinary Sciences
Research Article
Genotoxic and Clinicopathological Implications of Heliotropium Curassavicum Extracts in a Comparative Toxicological Study with Zinc Phosphide in Male Rats
Akram M. Eldidamony1, Gihan G Moustafa2, Hala M. I. Mead3, Mogeda M. Abdel hafez3, Bothina H. F. Omran4*
1Department of Chemistry, Faculty of Science, Zagazig University; 2Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Zagazig University; 3Department of Pest Physiology, Plant Protection Research Institute, ARC, Giza, Egypt; 4Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University.
Abstract | Exploring plant rodenticide is of great importance, as rodent damage has become a major ecological and environmental problem of many countries all over the world. The study was performed to elucidate the rodenticidal activity of acetonic, methanolic, and mixture of both extracts of Heliotropium curassavicum on adult male albino rats as alternatives to conventional rodenticides. The toxic effects of Heliotropium curassavicum acetonic, methanolic, and a mixture of both extracts at doses (625 and 825 mg/kg b.wt.), were evaluated compared to the toxicity of zinc phosphide (5 mg/kg b.wt.) under laboratory conditions, as well as control groups (tween 80, vegetable oil and distilled water) all administered by oral gavage for 28 days. Results showed a significant elevation in Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and alkaline phosphates (ALP) serum levels as affected by all tested treatments compared to control groups. On the other hand, a significant decline in α, β- Acetylcholinesterase (AchE), and Gamma-Aminobutyric Acid (GABA) levels was detected. Furthermore, there is a significant rise in the extent of micronucleus cell production in bone marrow smears of the treated rats compared to the control groups. Also, DNA fragmentation was noticed in hepatic and brain tissues after treatment with curassavicum plant extracts and zinc phosphide. Histopathological findings showed obvious perturbations in the liver and brain tissues that proved the previously mentioned results. Generally, our data reinforced that using the mixture of both extracts triggered more toxic impacts and rodenticidal activities than using each extract individually and could serve as a safer alternative of zinc phosphide.
Keywords | Heliotropium curassavicum; Zinc phosphide; Rodenticide; Micronucleus test; DNA fragmentation; Hepatotoxicity; Neurotoxicity.
Received | October 12, 2020; Accepted | October 20, 2020; Published | January 01, 2021
*Correspondence | Bothina Hassan Foad Omran, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University; Email: bosyomran82@yahoo.com
Citation | Eldidamony AM, Moustafa GG, Mead HMI, Abdel Hafez MM, Omran BHF (2021). Genotoxic and clinicopathological implications of heliotropium curassavicum extracts in a comparative toxicological study with zinc phosphide in male rats. Adv. Anim. Vet. Sci. 9(2): 203-214.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.203.214
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Omran et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Anticoagulant compounds such as zinc phosphide are generally effective against most rodent species which make them a benchmark for comparison with recently emerging rodenticides. Anticoagulant compounds commonly had significant risks on humans and the environment as well as the toxicity of several species and resistance to the target organisms (Prabhakar et al., 2019). Therefore, these problems provided an impetus to use poisonous natural materials known as biocide or green pesticides as an alternative safe supply to conventional pesticides (Gabr 2005). Various plant species have notorious pesticidal activities somewhere a lot of them are already identified with their chemical structure and effectiveness in lab settings to facilitate the rapid development of novel compounds (Stevenson et al., 2017).
Using plant extracts for pest control has several advantages in terms of preventing the development of insecticide resistance due to the usual presence of several bioactive compounds which act concertedly on both behavior and physiological processes (Isman, 2008). On the other hand, the limitations could be the unpredictable efficiency and lower toxic impact on target species.
Heliotropium is a large genus of the family Boraginaceae which consists of about 250-300 species that have numerous active ingredients especially pyrrolizidine alkaloids, terpenoids, and flavonoids. These phytochemicals enhance the insecticidal activities of plant extracts (Shaalan et al., 2005). Heliotropium curassavicum contains allelochemicals include alkaloids, phenolic acids, coumarins, terpenoids, and flavonoids which could inhibit the growing harmful weeds (Farrag et al., 2013). Moreover, it had been traditionally used for ulcers, wounds, local inflammations, erysipelas, edema, and bacterial infections (Katalinic et al., 2006). Generally, Heliotropium plants exhibited high toxicity as the over-consumption leads to venous occlusive disease, liver cirrhosis, hepatomegaly, splenomegaly, and death may result
(Lampe and McCann, 1985). However, they exhibit genotoxic effects due to the presence of pyrrolizidine alkaloids (Tamariz et al., 2018).
The current study aimed to comparatively evaluate the genotoxic and clinicopathological effects of Heliotropium curassavicum acetonic, methanolic, and a mixture of both extracts with zinc phosphide in adult male albino rats. In an attempt to introduce some Heliotropium curassavicum extracts as a new alternative and environmental-friendly weapon in rodent management.
Materials and methods
Plant Material Preparation
Complete H. curassavicum plant (Figure 1) was obtained from Sharquia Governorate, Egypt, (30°44’57.9”N 32°00’14.9”E) during April 2017 and identified at the Horticulture Research Institute, ARC, Egypt.
Chemical Reagents and Solvents
The chemicals and solvents used in this study were purchased from Gomhoria Co., Sharquia Governorate, Egypt.
Preparation of Extraction
The entire whole plant samples comprised of leaves, roots, stems, and flowers were cleaned from the dust and debris, cut into small pieces, and shade dried at room temperature (25 °C) for 7 days. The dried plant material was ground in a chopper into a crude powder. Extraction was performed as previously mentioned by Freedman et al. (1979) with slight modifications where the ground plant was soaked in the chosen solvents instead of using the soxhlet procedure. Four solvents varied in their polarity were used for serial extraction (hexane, acetone, methylene chloride, and methanol 70%). A sample of 1500 gm powder was soaked in the first solvent hexane for 3 days in brown-colored bottles, used as containers, and was provided with tight stoppers. After that, the bottles were intermittently shaken for 2 hrs. daily. The combined extract was filtered by using Whitman’s No.1 filter paper on the Buchner funnel. The concentration was done in rotary evaporator under reduced pressure at a temperature not exceeding (40-50 °C) in a water bath. The extracted powder was weighed and preserved under deep freezing. The marc was extracted in other solvents (acetone, methylene chloride, and methanol 70%, respectively) according to the previous steps. The identification of chemical compounds of both extracts was carried out by gas chromatography mass spectrometry (GC-MS) and the results were published in a prior study by Eldidamony et al. (2020).

Figure 1: Photograph of H. curassavicum.
Zinc Phosphide
Zinc phosphide (94 % active ingredient) was purchased from Kafr El-Zayat (Kz) Pesticide Company, Egypt.
Animals Used
Thirty-five mature male albino rats (120-180 gm) were assigned to 7 groups each one contained five rats, kept at a laboratory animal breeding unit (Faculty of Veterinary Medicine, Zagazig University), they were dosed orally. The rats were kept in clean metal cages, receiving routine diet and water during the whole experimental period. The light system was 12/12 hrs. dark/night cycle. Rats went on an accommodation period for 2 weeks before the procedures began. The experiment was terminated after 28 days from the first administration. All study design was approved by the ethics committee of the Faculty of Veterinary Medicine, Zagazig University, and the European Community Directive (86/609/EEC) on animal use and care were followed.
The rats were classified into seven groups each one contained five rats:
Group 1: was given 625 mg/kg b.wt.(1/20 of the LD50) of acetone plant extract (Eldidamony et al., 2020) prepared in tween 80.
Group 2: was given 825 mg/kg b.wt. (1/20 of the LD50) of methanol plant extract (Eldidamony et al., 2020) prepared in tween 80.
Group 3: was administered a mixture of both plant extracts with the same doses and rout.
Group4: was given 5 mg/kg b.wt. (1/10 of the LD50) of zinc phosphide (MacBean, 2012) prepared in vegetable oil.
Group 5, group 6, and group 7: were served as control groups and were administered 1ml of tween 80, vegetable oil, and distilled water, respectively.
All treatments were given once daily by oral gavage and the experiment was terminated after 28 days from the first administration, where the animals were sacrificed.
Biochemical assay
Collection of serum and blood samples: Blood samples were obtained under the ether anesthetic effect using a capillary glass tube from the retro-orbital plexus as described by Nemzek et al. (2001). Venous blood samples were gotten from each rat (3ml). The incubation of this blood took place at 37°C until clotting has occurred and then centrifuged at 3000 r.p.m. for fifteen minutes and the clear supernatant serum was kept at -20 0C till used it in the examination of biochemical parameters. Rats of each group were euthanized by cervical dislocation.
Determination of Biochemical Parameters
Both ALT and AST were measured colorimetrically as described by Reitman and Frankle (1957) The activity of ALP was determined following the method of Powell and Smith (1954) β and α Esterases enzymes were measured according to Van Asperen (1962). Evaluation of AchE and GABA were based on the method mentioned in the enclosed pamphlet of the manufacture Rat Gamma-Aminobutyric Acid ELISA Kit, Cat. No: MBS045103 and Rat Acetylcholinesterase ELISA Kit, Cat. No: MBS2501434.
Micronucleus Assay
Smears of bone marrow were prepared by the method of Schmid (1973), where 1000 polychromatic erythrocytes (PCEs) were scored per animal. Micronucleus with typical morphology described by Schmid (1975) was scored. The micronucleus was prepared according to procedures of Schmid (1976) and modified method of Agarwal and Chauhan (1993), Bone marrow cells were flushed out with 1 ml of fetal bovine serum and collected in a centrifuge tube.
The cell suspension was centrifuged at 1500 r.p.m for 10 min then the suspension was smeared, dried, and stained on a glass slide. The slides were observed under a light microscope using X100 oil immersion objective lens and X10 eyepieces. Scoring was performed according to Schmid (1975).
DNA fragmentation analysis of liver and brain tissues
DNA injury was evaluated by DNA fragmentation assay described by Bortner et al. (1995)
Tissue preparation for histopathological analysis: liver and brain tissues were routinely processed by dehydration in gradual ethanol (70–100%), cleared in xylene then paraffin-embedded tissues were cut 5 µm thick and then routinely stained with hematoxylin and eosin (H&E) dyes (Bancroft and Gamble, 2008).
Statistical Analysis
The significant differences were determined by analysis of variance (ANOVA). The significance of various treatments was evaluated by Duncan’s multiple range tests (p < 0.05). Data were subjected to statistical analyses using the software package Costat® Statistical Software (2005) version 6.311.
Results
Biochemical results
Effect of tested materials on liver enzymes: The current study proved that the daily treatment of adult male albino rats with H. curassavicum acetonic, methanolic, mixture of both extracts (625 and 825 mg/kg b.wt), respectively, and zinc phosphide (5 mg/kg b.wt) for 28 days showed a significant increase in serum ALT, AST and ALP levels in all treated groups compared to the control groups (Figure 2). The zinc phosphide treated group showed an obvious significant increase (p<0.05) followed by the co-administrated group then acetonic and methanolic groups.
Effect of acetonic and methanolic H. curassavicum extracts on α, β-esterase, (AchE), and γ- GABA: The obtained results revealed that treated groups with an acetonic, methanolic, mixture of both extracts and zinc phosphide showed a significant decrease e(p<0.05) in α-esterase, β-esterase, GABA levels and AchE activities compared to the control group (Figure 3).
As regarding α-esterase, the treated group with zinc phosphide showed an obvious significant decrease followed by a co-administrated and acetonic group as there was no significant difference between them, then the methanolic group as presented in.

Figure 2: ALT (U/ml), AST (U/ml) and ALP (U/ml) in tested extracts and zinc phosphide compared to control groups.
P** value <0.05 is considered significant. The significance of various treatments after ANOVA expressed as letters. Means with the same letter, the difference is not statistically significant. Means with a different letter, the difference is statistically significant.
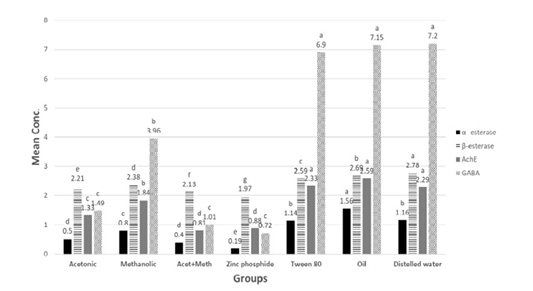
Figure 3: α –esterase (µg phenol/ml), β-esterase (µg phenol/ml), AchE (ng/ml) and GABA (mumol/L) in tested extracts and zinc phosphide compared to control groups.
P** value <0.05 is considered significant. The significance of various treatments after ANOVA expressed as letters. Means with the same letter, the difference is not statistically significant. Means with a different letter, the difference is statistically significant. NB: β-esterase mean values were divided by 100 to allow presenting all data on same graph.
Concerning β-esterase activities, the treated group with zinc phosphide showed a noticeable significant decrease followed by co-administrated then acetonic group and methanolic group.
However, in GABA levels there was a non-significant difference in groups treated with zinc phosphide, acetonic, and mixture of both extracts.
Concerning AchE activities, the treated group with zinc phosphide and co-administrated showed an obvious significant decrease followed by the acetonic and methanolic group.
Micronucleus test
Micronucleated polychromatic erythrocyte production in bone marrow cells of the treated animals and control groups: As regards the number of these micronuclei produced in the bone marrow cells of the treated animals they appeared as single- micronucleated cells or they may occur as bi-micronucleated cells. They also may present in multi-micronucleated cells as shown in (Figure 4). Concerning the shape, in the bone marrow smears of the examined material, the micronuclei appeared in the form of accumulated rounded masses and/or as dot-like structures. These micronuclei appeared also in other fields in the form of almond shape or they may be of rod-like as observed in (Figure 5).
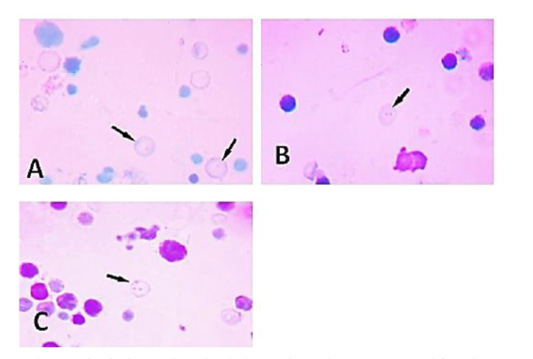
Figure 4: A micrograph of micronucleated polychromatic erythrocytes prepared from bone marrow cells of adult male rats treated showing variation in their number: (A) Single micronucleus. (B) Bi-micronuclei. (C) Multi-micronuclei.

Figure 5: A micrograph of micronucleated polychromatic erythrocytes prepared from bone marrow cells of adult male rats treated showing variation in their number (A) Almond shape (B) Rounded mass. (C) Rod-like structure. (D) Dot-like structure
The total number of micronuclei (MN) in the group treated with H. curassavicum acetonic, methanolic, mixture of both extracts and zinc phosphide were (94, 81, 116 and
Table 1: The effect of H. curassavicum acetonic, methanolic, mixture of both extracts and zinc phosphide on the frequencies of micronucleated polychromatic erythrocytes (MNPCEs) from bone marrow cells of adult male rats.
|
Groups
|
No of rats |
No. of scored cells 1000/ Rat |
+ MNPCEs |
P/N Ratio bone marrow activity (Mean/1000) |
||
| No. |
% |
Mean ± S.E. |
||||
| Acetonic extract | 3 | 3000 | 94 | 3.13 |
31.3±1.20bc |
0.031 |
| Methanolic extract | 3 | 3000 | 81 | 2.7 |
27.0±1.15c |
0.027 |
| Mixture of both extracts | 3 | 3000 | 116 | 3.8 |
38.6±0.88b |
0.038 |
| Zinc phosphide | 3 | 3000 | 171 | 5.7 |
57.0±0.87a |
0.057 |
| Tween 80 | 3 | 3000 | 26 | 0.87 |
8.6±1.85d |
0.0086 |
| Vegetable oil | 3 | 3000 | 24 | 0.8 |
8.0±1.52d |
0.008 |
| Distilled water | 3 | 3000 | 19 | 0.63 |
6.3±0.66d |
0.0063 |
SE: Standard Error.
Means within the same column carrying different superscripts are sig. different (P< 0.05)
171), respectively while, in control groups treated with tween 80, vegetable oil and distilled water were (26, 24 and 19), respectively.
Finally, the bone marrow activity which equals to the mean of MNPCEs per 1000 examined cells, measure the ability of bone marrow cells for the formation of micronuclei (MN) was reached 0.0086, 0.008, and 0.0063 in control groups (tween 80, vegetable oil and distilled water), respectively while it was 0.031, 0.027, 0.038 and 0.057 after treatment with (acetonic extract, methanolic extract, a mixture of both extracts and zinc phosphide) respectively. Therefore, the bone marrow activity was increased at all treated groups as compared with that of the control animals (Figure 6). The total number of micronuclei produced in bone marrow cells of the zinc phosphide-treated animals recorded a highly significant value followed by the co-treated group then acetonic and methanolic extracts treated group as compared to that of the control groups (Table 1).
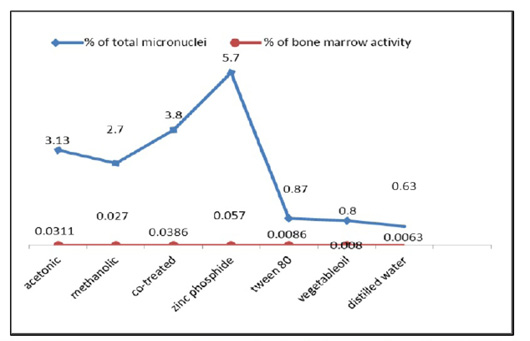
Figure 6: The frequency percentages of micronucleated polychromatic erythrocytes (MNPCEs) and the bone marrow activity from the bone marrow cells of control and treated animals.
Effect of H. curassavicum acetonic, methanolic, mixture of both extracts and zinc phosphide on liver and brain DNA fragmentation
DNA fragmentation in liver tissues of treated groups compared to control groups: Figure 7 showed an electrophoretic pattern of liver total genomic DNA fragmentation of treated groups with H. curassavicum acetonic, methanolic, and mix of both extracts at concentrations
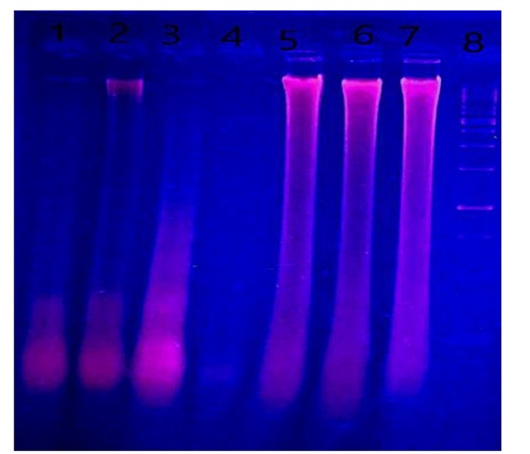
Figure 7: DNA fragmentation in liver of male albino rats orally administrated with H. curassavicum acetonic, methanolic, mixture of both extracts once daily at dose (625 and 825 mg/kg b.wt.), respectively and zinc phosphide at dose (5 mg/kg b.wt.) for 28 days. Lane 1: group treated with acetonic extract. Lane 2: group treated with methanolic extract. Lane 3: co-treated group. Lane 4: group treated with zinc phosphide. Lane 5: control group treated with tween 80, Lane 6: control group treated with vegetable oil, Lane 7: control group treated with distilled water. Lane 8: ladder.
(625 and 825 mg/kg b.wt.), respectively, and zinc phosphide at a dose (5 mg/kg b.wt.), as well as control groups (tween 80, vegetable oil and distilled water). There was severe liver DNA damage in all treated groups compared to the control groups, which was more obvious in liver tissues of zinc phosphide and H. curassavicum acetonic extract followed by methanolic and co-treated group compared to control groups.DNA damage represented by fragments migrated from the wells. On the contrary, control groups of tween 80, vegetable oil, and distilled water did not reveal the fragmentation of DNA.
DNA fragmentation in brain tissues of treated groups compared to control groups: As illustrated in (Figure 8) there was brain DNA damage in the group treated with a mixture of acetonic and methanolic extracts also in the group treated with zinc phosphide, but there was no fragmentation in acetonic and methanolic groups individually compared to control groups.
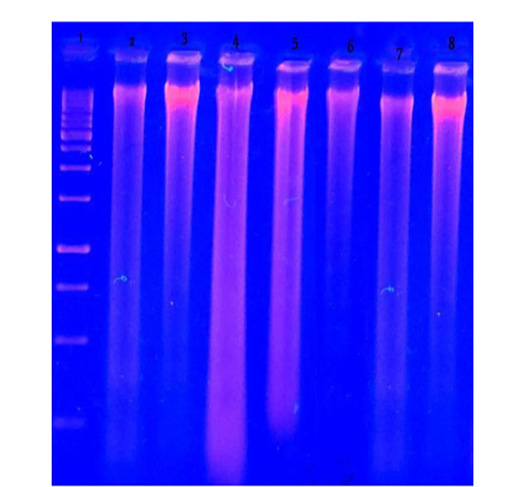
Figure 8: DNA fragmentation in brain of male albino rats orally administrated with H. curassavicum acetonic, methanolic, mixture of both extracts once daily at dose (625 and 825 mg/kg b.wt.) respectively and zinc phosphide at dose (5 mg/kg b.wt.) for 28 days. Lane 1: ladder. Lane 2: group treated with acetonic extract. Lane 3: group treated with methanolic extract. Lane 4: co-treated group. Lane 5: group treated with zinc phosphide. Lane 6: control group treated with tween 80. Lane 7: control group treated with vegetable oil. Lane 8: control group treated with distilled water.
Histopathological findings
Histopathological alterations in the liver tissues of rats treated with H. curassavicum acetonic, methanolic, mix of extracts and zinc phosphide, as well as control groups:
The liver in control groups treated with tween 80, vegetable oil, and distilled water showed normal histomorphological structures. However, the liver in the group treated
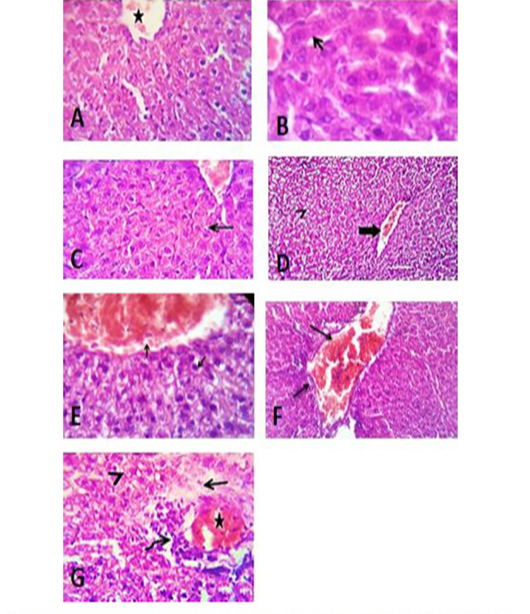
Figure 9: Liver sections of control group of tween 80 (A), vegetable oil (B) and distilled water (C) showing normal histological structure. (D) Photomicrograph of liver section of group treated with acetonic extract of H. curassavicum at dose (625 mg/kg b.wt.) for 28 days, revealed mild congestion of hepatic blood vessels (thick arrow) and degenerative changes in moderate number of hepatocytes (arrow head). (E) Photomicrograph of liver section of group treated with methanolic extract of H. curassavicum at dose (825 mg/kg b.wt.) for 28 days, showed congestion of hepatic blood vessels and degenerative changes in a moderate number of hepatocytes (arrows). (F) Photomicrograph of liver section of group treated with both acetonic and methanolic extracts of H. curassavicum at dose (625 and 825 mg/kg b.wt.), respectively for 28 days revealed severely congested portal blood vessels, portal lymphocytic infiltration and biliary hyperplasia (arrows). (G) Photomicrograph of liver section of group treated with zinc phosphide at dose (5 mg/kg b.wt.) for 28 days, showed moderate congestion of the hepatic blood vessels (stars), perivascular edema (arrow), round cells infiltration (curved arrow) and hydropic degeneration in large number of hepatocytes (arrow head). H&E. X 400.
with H. curassavicum acetonic extract (625 mg/kg b.wt) as well as, in the group treated with H. curassavicum methanolic extract (825 mg/kg b.wt.) revealed mild congestion of hepatic blood vessels and vacuolation and hydropic degeneration in a moderate number of hepatocytes. Meanwhile, the lesions in the liver of group co-treated with acetonic and methanolic extracts (625 and 825 mg/kg b.wt.), respectively were serious than each drug alone. The affected liver exhibited severely congested portal blood vessels, portal lymphocytic infiltration, biliary hyperplasia, and degenerative changes in a large number of hepatocytes. The liver showed moderate congestion of the portal blood vessels, perivascular edema, round cell infiltration, biliary proliferation, and hydropic degeneration in a large number of hepatocytes with early necrotic changes in some cells. While, the lesions in the liver of group treated zinc phosphide (5 mg/kg b.wt.) showed moderate congestion of the portal blood vessels, perivascular edema, round cells infiltration, biliary proliferation, and hydropic degeneration in a large number of hepatocytes with early necrotic changes in some cells as detected in (Figure 9).
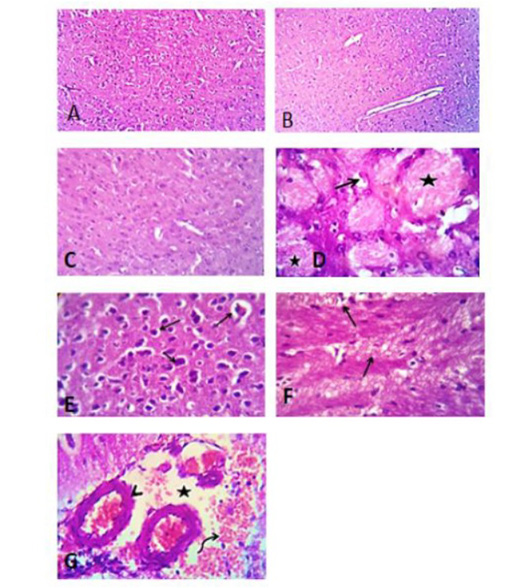
Figure 10: Photomicrograph of brain section of control groups treated with tween 80, vegetable oil and distilled water for 28 days showing apparently normal brain tissue (A), (B) and (C), H&E. X 100. (D) Photomicrograph of brain section of group treated with acetonic extract of H. curassavicum at dose (625 mg/kg b.wt.) for 28 days, revealed focal neuronal degeneration (open arrow) and coagulative necrosis of some axons (stars). (E) Photomicrograph of brain section of group treated with methanolic extract of H. curassavicum at dose (825 mg/kg b.wt.) for 28 days, showed Perivascular and perineural edema beside degeneration of moderate number of neurons in the cerebral cortex (arrows). (F) Photomicrograph of brain section of group treated with both acetonic and methanolic extracts of H. curassavicum at dose (625 and 825 mg/kg b.wt.), respectively for 28 days revealed perivascular edema, focal gliosis (open arrow) beside axonal degeneration and demylination (closed arrows). (G) Photomicrograph of brain section of group treated with zinc phosphide at dose (5 mg/kg b.wt.) for 28 days, showing perivascular edema (stars) and hemorrhage (curved arrow). The lateral ventricles showing dilatation with edema and congestion of the choroid plexuses (arrow heads). H&E. X 400.
Histopathological alterations in the brain tissues of rats treated with H. curassavicum acetonic, methanolic, mix of extracts and zinc phosphide, as well as control groups:
The brain of the control groups showed normal cerebral structures. On the other hand, the brain lesions of the group treated with the acetonic extract (625 mg/kg b.wt.) indicated focal neuronal degeneration, axonal degeneration demyelination, coagulative necrosis of some axons, and focal malacia. Also, the brain section of the group treated with the methanolic extract (825 mg/kg b.wt.) showed perivascular and perineural edema besides the degeneration of a moderate number of neurons in the cerebral cortex. While, the brain of the group treated with two extracts (625 and 825 mg/kg b.wt.), respectively revealed all previous lesions with focal gliosis. Furthermore, the brain tissues of the group treated with zinc phosphide (5 mg/kg b.wt.) showed neuronal and axonal degeneration with vacuolation, perivascular edema, and hemorrhage around the cerebral blood vessels besides demyelination of the axonal sheaths in most parts of the cerebral cortex as observed in (Figure 10).
Discussion
Up till now chemical rodenticide continued to be used widely as it has the most significant role among all the rodent control methods; whereas, it produces heavy environmental pollution and maybe poisoning. Using plants as a rodenticide has drawn lots of attention since it does not cause damage to the environment and won’t arouse resistance and it is also easy to be degraded by nature. In the present study, Heliotropium curassavicum extracts have been presented as a novel safer substitute for conventional rodenticide as zinc phosphide. Hepatic and brain toxic consequences of both Heliotropium curassavicum extracts and zinc phosphide exposure have been investigated.
The liver is a vital homeostatic organ in the body as the toxic compounds accumulate in the liver and detoxified. Liver damage caused by toxic substances could be confirmed by the assessment of liver function biomarkers such as AST, ALT, and ALP; these enzymes are sensitive indicators for the degree of hepatic injury according to Patrick-Iwuanyanwu et al. (2012). Our results showed that zinc phosphide caused a significant rise in ALT, AST, and ALP levels which agreed with Karanth and Nayyar (2003) who reported that zinc phosphide produces phosphine gas by reacting with gastric fluids and acids. The buildup of this gas leads to several system injuries, inhibiting the activity of respiratory enzymes with mitochondrial dysfunction, increasing free radicals resulting in lipid peroxidation. Besides, Venugopal et al. (2018) reported that ingestion yellow phosphorus caused liver damage with significant changes in AST and ALT. Moreover, Karanth and Nayyar (2003) who concluded that hepatotoxicity following acute poisoning with rodenticides was combined with an increase in ALT 1055U/L, AST 824U/L, ALP 13 U/L. Also, Emara et al. (2018) observed that acute and hyper-acute zinc phosphide toxicity at doses 35 mg/kg and 60 mg/kg induced a marked increase in liver enzymes.
Our data illustrated that the co-treated group revealed a significant increase in ALT, AST, and ALP followed by the acetonic extract then the methanolic extract. This elevation could be attributed to the activities of flavonoids, alkaloids, tannins, and phenols of the plant. This was supported by Hassan et al. (2007) who reported that the tannins, saponins, glycosides, and alkaloids in the leaves of Erythrophleum africanum accounted for the significant increase in ALP and AST after sub-acute administration of the extract for 28 days at dose 3000 and 2000-3000 mg/kg b.wt. Also, in the same approach, Adeoye and Oyedapo (2004) concluded that alkaloids isolated from E. guineense induced elevation in the activity of plasma ALT, AST, and ALP. As well, that studied by Tion et al. (2018) recorded that phytochemical screening of Abrus precatorius showed alkaloids, flavonoids, tannins, saponins, and reducing sugars in both ethanolic and aqueous extracts. Biochemistry revealed significantly increased alkaline phosphatase in mice treated with 1/10 (18.75 mg/Kg) of LD50 of methanolic and ethanolic seed extracts, for a month. Also, our results were supported by the finding of Kyei et al. (2015) who recorded that frequent exposure of the rats to different doses of aqueous extract H.indicum over four weeks indicated concomitant elevation levels of ALP.
In contrary to our results, Yusufoglu et al. (2013) evaluated the effect of Taraxacum serotinum and H. europaeum extracts on the reproductive functions of male rats at doses 100, 200, and 400 mg/kg for 7 weeks. They observed that the oral administration of both extracts had no significant effect on the serum activities of AST and ALT. Besides, Kyei et al. (2015) revealed that rats treated with H. indicum aqueous extract at all dose levels showed significant reductions in alkaline phosphatase (ALP) levels. This may be returned to the difference in the examined plant species and dose.
The results of the current study revealed that the rats treated with zinc phosphide showed a highly significant decrease in serum levels of α, β-esterases, AchE, and GABA. These results were supported by Mittra et al. (2001) who concluded that aluminum phosphide poisoning causes cholinesterase inhibition and responds to treatment with atropine and pralidoxime. In the same contest, Al-Azzawi et al. (1990) recorded neurotoxic behavior of phosphine intoxication as phosphine gas could be related to exaggerated acetylcholine neurotransmission, which is attributed to the suppression of acetylcholine esterase and the resultant buildup of acetylcholine. Moreover, Sciuto et al. (2016) stated that the metallic phosphides like aluminum, magnesium, and zinc phosphides induced cytotoxicity, oxidative stress, cholinesterase inhibition, hepatotoxicity, neurotoxicity, and generally disturbed metabolism.
The aforementioned data revealed that the H. curassavicum contains flavonoids, alkaloids, tannins, and phenols; the presence of such phytoconstituents may be responsible for the decrease revealed in α, β-esterases, AchE, and GABA serum levels of mature male albino rats. These findings were supported by Wink (2003) who found various alkaloids could restrain the enzymatic breaking down of neurotransmitters. Moreover, Wanchun et al. (1997) indicated that alkaloids from plants such as cytisine sophoramine, sophoradine, sophocarpine, aloperine, cytisine could inhibit the activity of AchE, α-NA esterase, α-NA carboxylesterase, and esterase isozyme as well. This result was in parallel with Mutschler et al. (2008) who reported that neuroreceptors are another prime target for numerous alkaloids, which have a structure resembling the endogenous neurotransmitters, for example, acetylcholine, dopamine, nor-adrenaline, serotonin, adrenaline, GABA. The neuroactivity of these alkaloids could be agonistic, over stimulating the neuro-receptor, or as antagonistic with blocking effects. Our results revealed the presence of the flavonoid kaempferol in the methanolic extract; it was identified as esterase inhibitors according to Li et al. (2007) Similarly, Xie et al. (2014) reported that flavonoids produce inhibitory effects on AchE as the hydroxyl groups in flavonoids are favorable for inhibiting it.
Regarding genotoxicity by zinc phosphide, the above-mentioned results agreed with Barbosa et al. (1994) who recorded that phosphine is a genotoxic compound as it produced a rise in micronucleus incidence in the bone marrow and splenic lymphocyte of rat on exposure to maximum levels. Different bioassays: bone marrow chromosome aberration, micronucleus, and spermatic aberrations could be utilized to evaluate the genotoxic potential of zinc in mice in vivo (Pal and Bhunya, 1995). The results showed a dose-dependent elevation in the rate of micronuclei in the bone marrow cells. Moreover, Türkez and Toğar (2013) evaluated the genetic and oxidative damages of aluminum phosphide in rats at a dose 4 mg/kg injected intra-peritoneally for 14 successive days. They found that aluminum phosphide induced a rise in chromosomal aberrations and micronucleus frequencies.
Moreover, our results came in harmony with Carballo et al. (1992) who analyzed the genotoxic effect produced in vitro by H. curassavicum by studying the chromosomal aberrations (CA), mitotic index (MI), and anaphase delay (AD) in the Chinese hamster ovary (CHO) cell line. They found a marked elevated percent of abnormal metaphases and total aberrations. The MI, as well as the AD, impacted the cell cycle. The toxic effect could be attributed to the presence of pyrrolizidine alkaloids and their N-oxides. Also, Tamariz et al. (2018) recorded that some species of Heliotropium exhibit genotoxic effects due to the presence of pyrrolizidine alkaloids such as H. curassavicum and H. crassifolium. The previous data revealed the presence of colchicine in the methanolic extract of H. curassavicum, thus strengthening our results as it has been recorded as a part of a disparate group of chemicals that are capable of altering genetic materials by disrupting the mitotic spindle during the cell division, so multiple sets of chromosome (<2) within a single cell according to Barry (2000).
Concerning DNA fragmentation results, the previous data could be attributed to the reports of Muid et al. (2012) who proved the ability of zinc phosphide to prompt DNA break in blood cells of the fowl by comet assay. Furthermore, the observed genotoxicity was found to be dose-specific and dependent. Also, DNA injury exhibited a positive correlation to the duration of exposure. Moreover, our results agreed with those of El Okle et al. (2018) who investigated the hepatic DNA fragmentation in rats killed by slaughtering, electrocution, drowning, and zinc phosphide poisoning, respectively. Liver samples were collected at zero, 2, 6, 12, and 24 hrs. postmortem time points. The poisoning by zinc phosphide enhanced the rate of DNA degradation over all other groups. Hepatic DNA of zinc phosphide-intoxicated rats showed the most extensive and rapid damage among all experimental groups. The ability of zinc phosphide to induce hepatic DNA damage seems to be linked to the over-production of ROS.
These findings were in parallel with Tamariz et al. (2018) who illustrated that some species of the genus Heliotropium exhibit genotoxic effects such as H. curassavicum and H. crassifolium related to pyrrolizidine alkaloids. The aforementioned results revealed that the acetonic extract of H. curassavicum contains benzene derivatives that induce myelosuppression, aryl hydrocarbon receptor (AhR) agonist, induction of chromosomal aberrations, immune dysfunction (TNF-α, INF-γ), initiation of redox cycling, protein alkylation (tubulin, histones, topoisomerases), and growth factor inhibition according to Ramaiah et al. (2013).
Regarding the histopathological finding of the liver and the brain tissues, the present study results came in harmony with finding recorded by Lampe and McCann (1985) who illustrated that excessive exposure to Heliotropium plants could lead to hepatic veno-occlusive syndrome along with pain and ascetic fluids accumulation in the abdomen. Besides, hepatic cirrhosis, hepatomegaly, splenomegaly, and end fatally due to deficiency of definite cure in such cases. These results were consistent with Huxtable (1989) who reported that H. curassavicum plant contains pyrrolizidine alkaloids of the noncyclic diester type. Overconsumption of such a plant may cause veno-occlusive disease of the liver with a hepatic vein thrombosis. Moreover, Kyei et al. (2015) revealed that rats treated with H. indicum at a dose of 100 mg/kg suffered from some histological deformation in the form of edemas and renal degenerative changes with lost glomeruli, edematous appearance with activation of Kupffer cell, leucocytic infiltrate within hepatic sinusoid. Also, the data described above were in agreement with findings recorded by Cullen and Stalker (2016) who reported that long-term consumption of Heliotropium leads to hepatic fibrosis and atrophy in absence of regeneration nodules due to the presence of pyrrolizidine alkaloids which also cause severe focal to huge necrotic changes in hepatic lobules. Furthermore, repeated exposure to such alkaloids could result in atrophied liver and nodular regeneration. Besides, our results are supported by Karanth and Nayyar (2003) who proposed that inorganic phosphorus is a potent hepatotoxin as a hepatic failure following the ingestion of a phosphorus-based rodenticide was observed. Moreover, liver sections showed piecemeal necrosis, fibrosis, collection of inflammatory cells, and hydropic change. Also, Dua et al. (2010) demonstrated that phosphine gas exposure exhibits a histopathologic deformation within the brain cells of animals that was fed phosphide residue polluted cowpea. Thus, come back to the inhibition of the cytochrome oxidase which turn down oxygen uptake along with diminished ATP production and finally led to cutting off cell energy and activate anaerobic glucose pathway, thus resulting in a marked lowering of plasma glucose level. This down-regulation of ATP production with accompanying hypoglycemia could compromise the energetic state of brain cells besides disturbed glucose homeostasis. Moreover, the results were supported by Alex (2009) who showed that zinc phosphide has toxic effects including cardiac cytotoxicity, dehydration, adrenal gland injury, DIC, liver necrosis, and kidney malfunction. Besides, the present study concurs with the findings of Emara et al. (2018) who evaluated the effect of acute and hyper-acute zinc phosphide toxicity in rats. The histopathological examination revealed vacuolation of hepatocytes, a diffuse advanced stage of hepatic steatosis, and apoptosis in the group of acute toxicity, while the hyper-acute group showed an increased cellular apoptosis and bile duct epithelium hyperplasia.
Conclusion
From the results of the current study, it has been determined that Heliotropium curassavicum (acetonic, methanolic, and mixture of both) extracts possess promising rodenticidal activity with less environmental toxic impact in comparison to conventional rodenticidal as zinc phosphide. Treatment with the plant extracts as well as zinc phosphide carries toxic effects on the liver and brain tissues of the rats, which is evident by biochemical parameters, micronucleus cell production, DNA fragmentation, and histopathological changes. Also, a mixture of both acetonic and methanolic extracts caused more toxic impacts than using each extract individually. Further toxicity studies need to be undertaken to elucidate the rodenticidal activity of herbal extracts and their underlying mechanisms, target pest species possible environmental, and human effects.
acknowledgements
The authors gratefully acknowledge Prof. Dr.Sayed Elattar, Professor of Pathology, Faculty of Veterinary Medicine, Zagazig University, for his help in histopathological tissue examination. And Dr. Samah N. Elshafiey for her assistance in the biochemical examination
Conflict of Interest
The authors declared no conflicts of interest.
Authors Contribution
Prof. Akram M. Eldidamony: Contribute to planning, writing, editing, revising, and interpretation of the data.
Prof. Gihan G. Moustafa: Contribute to planning, editing, revising, and interpretation of the data.
Prof. Hala M. I. Mead: Contribute to planning, writing, editing, revising, and interpretation of the data.
Dr. Mogeda M. Abdel Hafez: Contribute to planning, writing, editing, revising, and interpretation of the data.
Dr. Bothina H. F. Omran (Corresponding Author: Contribute to planning, writing, editing, revising, and interpretation of the data.
Authors contributed equally to this work each in his area of expertise
References





