Advances in Animal and Veterinary Sciences
Case Report
Malignant Cutaneous Melanoma in a Shih Tzu Mix: A Case Report
Rasmini Wickrama Surendre1, Mohd Farhan Hanif Reduan1*, Sabri Jasni1, Sujey Kumar Rajendren2, Nadiah Syuhada Roslan3, Intan Noor Aina Kamaruzaman1, Nurshahirah Shaharulnizim2, Muhammad Luqman Nordin2
1Department of Paraclinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia; 2Department of Clinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia; 3Universiti Malaysia Kelantan Veterinary Clinic, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia.
Abstract | Melanoma or malignant melanoma is an abnormal production of melanocytes in a dysregulated manner leading to the formation of a nodule, mass or other types of lesion. In this report, we describe the rare occurrence of malignant cutaneous melanoma as melanomas of haired skin origin are generally known to be benign and less aggressive compared to its mucocutaneous counterpart. A thirteen-year old, spayed female Shih Tzu Mix was presented to Universiti Malaysia Kelantan Veterinary Clinic with a history of a bleeding mass on the left scapular region. Upon physical examination of the integumentary system, there were generalized brownish to greyish and black macules of varying sizes as well as soft, black nodules with a smooth surface located at the right lateral trunk region and at the caudal-proximal region of the tarsal joint. The mass on the left scapular region appeared alopecic, firm and cauliflower-like with an ulcerating centre. Complete blood count revealed a mild to moderate lymphopenia and neutrophilic granulocytosis. The cytological evaluation revealed pleomorphic neoplastic cells characterized by spindle to round phenotypes, having dark, dust-like intracytoplasmic melanin granules with multiple prominent nucleoli and mitotic figures. The patient was treated via surgical excision of the ulcerating tumour. Histopathological examination of the excisional biopsy revealed pagetoid melanocytosis, spindle and round neoplastic cells with high mitotic index, marked tumour lymphoplasmacytic infiltration and intracytoplasmic melanin pigments. Discretely scattered neoplastic melanocytes and melanin pigments were also observed within the subcutaneous layer, indicating tumour infiltration. In conclusion, the diagnosis of cutaneous melanoma was made based on cytological and histopathological findings and treatment was done via surgical excision.
Keywords | Cutaneous melanoma, Malignant, Cytology, Histopathology, Surgical excision
Received | August 16, 2020; Accepted | November 27, 2020; Published | December 01, 2020
*Correspondence | Mohd Farhan Hanif Reduan, Department of Paraclinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia; Email: farhan.h@umk.edu.my
Citation | Surendre RW, Reduan MFH, Jasni S, Rajendren SK, Roslan NS, Kamaruzaman INA, Shaharulnizim N, Nordin ML (2021). Malignant cutaneous melanoma in a shih tzu mix: A case report. J. Anim. Health Prod. 9(1): 47-51.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.1.47.51
ISSN | 2308-2801
Copyright © 2021 Surendre et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Epidemiologically according to Withrow et al. (2013), melanoma accounts for 5.6% of all common skin neoplasms in dogs. Within melanoma, malignant melanoma being more prevalent (76.9%) compared to its benign counterpart, melanocytoma (23.1%) (Teixeira et al., 2010). There are four types of melanomas; oral melanoma with a high prevalence of 62%, cutaneous melanoma with a prevalence of 27%, digital or subungual melanoma of 10% and the least prevalent is ocular melanoma of 1% (Gillard et al., 2014). As a general rule, melanomas arising from haired skin are considered benign whereas those of mucocutaneous origin are typically malignant as numerous literature has suggested (Gillard et al., 2014; Moulton, 2017; Nishiya et al., 2016; Smith et al., 2002). However, here we are reporting the rare occurrence of malignant cutaneous melanoma that behaved aggressively.
MATERIALS AND METHODS
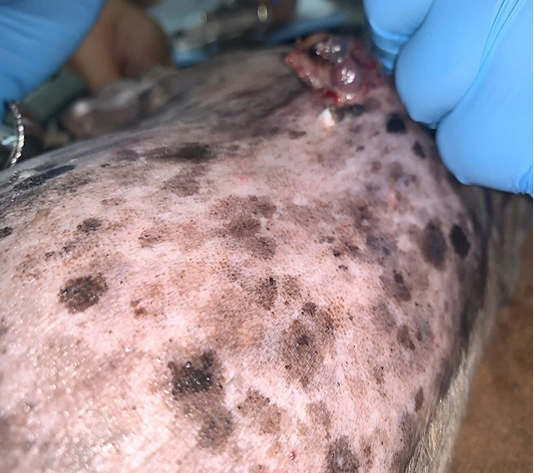
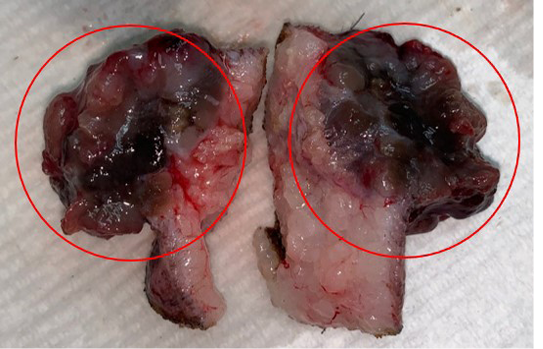
Lele, a spayed, 13-year old, female Shih Tzu Mix was presented to Universiti Malaysia Kelantan Veterinary Clinic with a mass at the left shoulder. Upon physical examination of the integumentary system, there were generalized brown-greyish and blackish macules of varying sizes ranging from 0.2-1.0 cm with irregular borders (Figure 1). There were also focal, soft black nodules with a smooth surface of 1 cm in size observed at the right lateral trunk region (Figure 2) and at the caudal-proximal region of the tarsal joint. The mass at the left scapular region was alopecic, firm, cauliflower-like with an ulcerating centre, measuring 2.5 x 2.5 cm in size (Figure 3). Complete blood count (CBC) showed an inflammatory leukogram with mild neutrophilic granulocytosis (10.9 x 103/μL, reference: 2.0-8.0 x 103/μL) and mild absolute lymphopenia (0.7 x 103 /μL, reference: 1.0-5.0 x 103/μL). Cytology evaluation of the mass revealed pleomorphic neoplastic cells that were characterized by spindle to round phenotypes, having dark, dust-like intracytoplasmic melanin granules with multiple prominent nucleoli and mitotic figures (Figure 4). The dog was tentatively diagnosed with cutaneous melanoma following the clinical findings and clinical pathology results. Surgical excision of the tumour was performed and the excised tissue was fixed with 10% buffered formalin. The samples were trimmed with 0.5 cm thickness and placed into histological cassettes which were then immersed in 10% formalin overnight. The fixed tissues were then embedded into paraffin wax, sectioned at 4 μm thickness and stained with Haematoxylin and Eosin (H and E) (Aliyu et al., 2020; Reduan et al., 2020).

Figure 2: Focal and soft black nodules with a smooth surface of 1 cm in size observed at the right lateral trunk region.
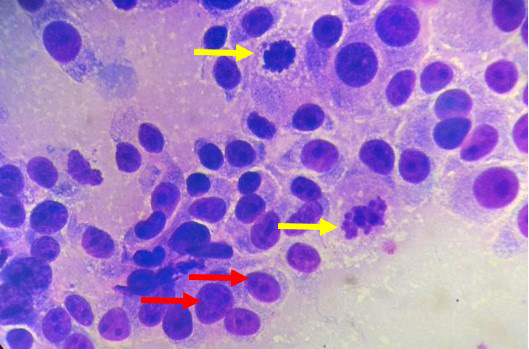
Figure 4: Pleomorphism characterized by a mixture of spindle and round cells and presence of multiple nucleoli (red arrows), mitotic figures (yellow arrows) and intracytoplasmic melanin granules (H and E, 1000X).
RESULTS AND DISCUSSION
Grossly, the tumour had a cauliflower-like appearance and was brownish to black in colour externally as well as on the cut surface (Figure 3). The pigmented growth is a typical appearance of melanomas as seen in another case report by Veena et al. (2012) whereby the mass was also pigmented and solitary. On the contrary, Palanivelu et al. (2013) reported multiple cutaneous masses on different locations on the body that were both pigmented and unpigmented or amelanotic.
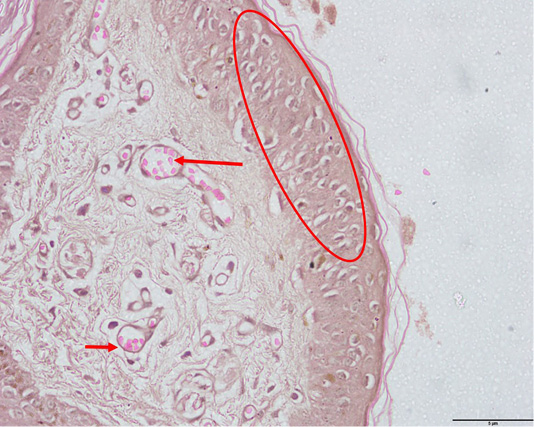
Microscopically, the neoplastic cells demonstrated pleomorphism characterized by a mixture spindle and round cell phenotypes and a high mitotic index (>3 mitoses/ 10 high power fields) were observed within the dermis layer (Figure 5). Tumours with a high mitotic count are typically associated with aggressive behaviour and a poorer prognosis as described by Moulton (2017). Intracytoplasmic melanin granules were also observed throughout the epidermis, dermis and the subcutaneous layers (Figure 5). The aggregates of melanin pigment is also a common feature in the case reports by Veena et al. (2012) and Palanivelu et al. (2013). Furthermore, individual melanocytes were randomly scattered throughout the superficial epidermis, indicating pagetoid melanocytosis (Petronic-Rosic et al., 2004; Smith et al., 2002) which is a feature not observed with melanocytomas (Figure 6). There were also extensive spindle-shaped and round cells in the subcutaneous layer, indicating tumour invasion to the said layer. In addition to that, the histopathological analysis also revealed a moderate to marked tumour lymphoplasmacytic infiltration within the dermis suggestive of inflammation. Results of histopathological findings confirmed the case of cutaneous melanoma. As mentioned previously, numerous studies have found that cutaneous melanocytic tumours are typically benign however in the present case it was malignant. This can be attributed to various intrinsic and extrinsic factors. For example, the presence of the inflammatory cells release reactive oxygen species (ROS) which are mutagenic for nearby neoplastic cells, therefore contributing to their genetic evolution towards states of heightened malignancy (Grivennikov et al., 2010). In addition, geriatric patients have decreased number of melanocytes in the basal layer of the epidermis leading to reduced pigmentation (Mills, 2012). The patient’s body coat was also trimmed short. All these conditions can predispose the patient to increased ultraviolet (UV) exposure.
Tumours of melanocytic origin are great imitators of other tumours as they can show features of round cells, epithelial cells or even mesenchymal cells and often, a mixture of all three of these morphological appearances will be present from a single tumour (Cowell and Valenciano, 2014). Other important histological features of melanomas include the presence of sheets or nests of neoplastic melanocytes at the basal portion of the epidermis or intraepidermis which were not observed in the present report (Gross et al., 2005). There would also be grey to dark, dust-like intracytoplasmic melanin granules in cells on cytology that help determine the tumour is melanocytic (Raskin and Meyer, 2016). These melanin granules are produced within lysosome-related organelles of melanocytes called melanosomes. However, the degree of pigmentation is highly variable as amelanocytic melanomas lack discernible melanin pigment.

Figure 5: Pleomorphism with spindle and round cells, anisokaryosis and mitotic figures (yellow arrows) as well as intracytoplasmic melanin pigments observed within the dermis (red arrows) (H and E, 400X).
Other diagnostics of melanoma include the use of special stains such as Fontana Masson which stains melanin granules black and immunohistochemistry using markers such as Melan A which is a melanocyte lineage-specific marker (Ramos-Vara and Miller, 2011). Moreover, it is also pivotal to carry out a metastasis check by examining the regional lymph nodes as well as obtaining thoracic radiographs to assess for pulmonary and thoracic lymph nodes metastasis. Abdominal ultrasonography should also be conducted to assess metastasis to the visceral organs and lymph nodes. A metastasis check would aid in the staging of the tumour which in turn, helps construct a more accurate prognosis. Metastasis of melanoma can occur via hematogenous and lymphatic routes therefore it is common for the tumour to spread to regional lymph nodes, lung and liver as these organs are highly vascularized. Brain and gastrointestinal tract metastases have also been reported (Kesdangsakonwut et al., 2015). A complete blood count and serum biochemistry to assess for liver and kidney functions, as well as paraneoplastic syndromes such as hypercalcemia of malignancy and hypoglycemia, should be conducted. In this case, the neutrophilic granulocytosis noted in CBC may be due to inflammation or as a result of cytokines such as granulocyte-colony stimulating factor (G-CSF) produced by the tumour to help create a microenvironment suitable for its own growth and progression (Withrow et al., 2013). The lymphopenia on the other hand, may be due to increased susceptibility of lymphocytes to apoptosis elicited by the tumour itself (Dworacki et al., 2001) and/or due to the extravasation of lymphocytes from the circulating pool to the surrounding tissues, infiltrating the tumour whereby they are called tumour infiltrating lymphocytes (TILs) (Davis et al., 2019; Zoladz, 2019).
The ideal treatment for malignant melanoma involves a combination of local control which can be achieved through surgical excision and/or radiotherapy (Laver et al., 2018) and systemic tumour control via chemotherapy or immunotherapy to delay metastasis (Henry and Higginbotham, 2010). In this case, wide excision preferably 2-3 cm margin with the subcutaneous tissue excised is the much preferable treatment option to ensure clean surgical margins. The evidence of an incomplete surgical excision is supported by the histopathological findings of tumour invasion into the subcutaneous layer, which was not removed during surgery as only 1 cm of tissue margin was excised. In the case of incomplete excision, the patient should be monitored intensively for recurrence, undergo post-operative adjuvant therapy or immediate re-excision of the surgical wound bed should be performed (Kudnig and Seguin, 2012). Other treatment alternatives include hypofractionated radiotherapy (Freeman et al., 2003) which was unavailable in this case and chemotherapy typically using alkylating agents such as carboplatin or cisplatin. However, response rates to chemotherapy is poor, ranging from only 8 to 28% due to chemoresistance of melanoma (Bergman et al., 2003).
The limitations of this study should be addressed. A biopsy or fine-needle aspiration of sentinel lymph nodes as well as a thoracic radiograph should have been taken considering the aggressive nature of the tumour. This would aid in the staging of the melanoma according to the World Health Organization TNM-Staging Scheme for dogs which is pivotal in constructing a more accurate prognosis. Furthermore, the authors suggest that more research should be conducted to discover more accessible and less costly alternative treatment for the systemic control of melanoma. Currently, there has been a great interest in developing immunotherapy as a reliable treatment option to help delay progression of metastasis and following this, the canine melanoma vaccine was produced (Wilson-Robles, 2013). Unfortunately, this vaccine is not widely available, is costly and requires a veterinary oncologist to be administered.
In conclusion, the diagnosis of cutaneous melanoma was made based on cytological and histopathological findings and treatment was done via surgical excision. Cutaneous melanoma is a fairly common skin neoplasm in dogs that can either behave benignly or malignantly. In more aggressive melanomas, local control with surgical resection supported by adjuvant therapy is necessary to enhance survival times.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Veterinary Officers of UMK Veterinary Clinic and laboratory staff of Clinical Pathology and Histopathology, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan for their technical assistance during the time of handling this case.
AUTHORS CONTRIBUTION
All authors contributed equally and approved the final manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES