Advances in Animal and Veterinary Sciences
Research Article
Specific Prophylaxis of Viral Diseases of Calves with Diarrhea Syndrome Under Associative Clinical Course
Alfia Andreeva*, Oleg Altynbekov, Oksana Nikolaeva, Chulpan Galieva, Ruzil Avzalov
Department of Infectious Diseases, Zoohygiene and Veterinary Sanitary Inspection, Federal State Budgetary Educational Establishment of Higher Education “Bashkir State Agrarian University”, Ufa, Russian Federation.
Abstract | The research aim was to correct colostral immunity using the immunostimulator “Immunat” in connection with vaccination for improving the young animals safety and increasing body weight gain. 20 pregnant cows of black-and-white breed, as well as calves born to them from birth to two months of age were the research object. Blood serum samples serological tests for the presence of anti-rotavs, anti-coronavirus, and anti-diarrhea virus antibodies prior to vaccination, 40 days before calving, 20 days before calving, before calving, and in the colostrum of the first milk yield were performed in dams. Serological studies of blood serum samples taken from calves born to vaccinated cows were performed before colostrum intake, one day after its first intake, as well as on the 7th ,14th , 21st days of the calf’s life and at the age of 30 and 60 days for the anti-rotavirus, anti-coronavirus, and anti-diarrhea virus antibodies presence; quantitative determination of the concentration of immunoglobulins A, M, G in animal sera on the 25th, 35th, 65th and 75th days of the calf’s life was carried out. It was found that the immunostimulator “Immunat” application to pregnant cows before vaccination and to newborn calves increases the vaccination effectiveness against associative infections of young animals resulting in the increase in the immunoglobulins content in colostrum and serum, as well as in the increase in antibody titer against rotavirus, coronavirus, and diarrhea virus. The use of the drug “Immunat” improves the calves safety in the postnatal period and drives up growth and development.
Keywords | Colostral immunity, Antibody titer, Immunoglobulins A, M, G, Rotavirus, Coronavirus.
Received | October 12, 2020; Accepted | October 20, 2020; Published | December 10, 2020
*Correspondence | Alfia Abdreeva, Department of Infectious Diseases, Zoohygiene and Veterinary Sanitary Inspection, Federal State Budgetary Educational Establishment of Higher Education “Bashkir State Agrarian University”, Ufa, Russian Federation; Email: alfia_andreeva11@rambler.ru
Citation | Andreeva A, Altynbekov O, Nikolaeva O, Galieva C, Avzalov R (2021). Specific prophylaxis of viral diseases of calves with diarrhea syndrome under associative clinical course. Adv. Anim. Vet. Sci. 9(1): 103-110.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.103.110
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Andreeva et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
During prenatal development, the calf does not receive the mother’s immunoglobulins since antibodies are filtered out by the placental barrier in the bloodstream that feeds the calf in the womb and do not reach the embryo (Baintner, 2007, Barrington, 2001). At birth, calves have a physiological immunodeficiency caused by the absence of immunoglobulins in the blood (Al-Alo et al., 2018, Andreeva et al., 2018). All calves are hypogammaglobulinemic at birth. Delivery of immunoglobulins into the body of calves is accomplishable by colostral route only (Ivanov et al., 2018, Ort et al., 2018). The colostral immunity stress directly depends on the level of maternal immunoglobulins absorption through the intestinal mucosa of a newborn calf. Numerous studies have shown that the most intensive absorption of globulins occurs during the first 6-12 hours after birth, while there are no changes in their structure and immunobiochemical properties.
The duration of colostral immunity is determined by the half – life of immunoglobulins circulating in the bloodstream: for immunoglobulin A, it is 4-6 days, for immunoglobulin G-10-21 days, for immunoglobulin M – 4 days. As a rule, the protective effect of colostral immunity in a calf ends by the age of one month. However, together with the colostral immunoglobulins reduction, adaptive immunity is built up in a calf from two weeks of age (Janjatović et al., 2008; Usachev and Polyakov, 2007).
Deficiency or insufficient number of colostral immunoglobulins leads to the decrease in weight gain and to the increase in susceptibility to gastro-intestinal infections. Thereupon, very early cases of morbidity and mortality are typical (Chase et al., 2008, Kirkpatrick et al., 2001, Safonov, 2020).
The short postnatal period, during which colostral immunoglobulins can be transferred to the system of a newborn animal, is simultaneous with the rapid decrease in gammaglobulins in successive portions of milk produced from the dam (Ellis et al., 2001, Pearson et al., 2019). For the protection of a young animal, the duration of the presence and concentration of maternal antibodies in its system are of great importance since they must enter its body before the evolvement of its own immune apparatus (Han et al., 2019, Kampen et al., 2006).
The immunological value of colostrum can be increased by direct vaccination of cows with appropriate antigens which leads to an additional accumulation of specific antibodies against infectious agents (Hodgins and Shewen, 2012). Immune colostrum usually has a higher level of immunoglobulins than normal (Gregorko et al., 1989; Raimondi et al., 2007, Usachev and Polyakov, 2007). In order to obtain it, cows are vaccinated in the second half of gestation (Hodgins and Shewen, 2012, Silva et al., 2018).
Studies published in the United States describe the results of cattle’s experimental infection with bovine viral diarrhea subgenotypes after vaccination. Vaccines provided protection against heterologous strains in the range from 100% to partial, but statistically reliable. The immunity duration provided by vaccines has also been reported (Fulton, 2015).
European scientists working under Bosco Cowley (2014) studied seroprevalence aspects of bovine herpesvirus 1 and bovine viral diarrhea virus and vaccination of dairy herds in Northern Ireland. They proved that true seroprevalence of unvaccinated herbs in Northern Ireland to bovine herpesvirus 1 is 77.3%, and to bovine diarrhea virus it is 98.4%. The authors state that these studies are necessary to reveal baseline conditions and to assess the progress of current and future infectious disease control and eradication programs (Bosco Cowley et al., 2014).
When immunizing dams, it has been found that the increase in the number of mediating antibodies and the protection of a susceptible animal go side by side (Ridpath et al., 2003, Silva et al., 2018).
When immunizing dams, colostrum with specific antibodies is produced to pass specific protection to newborn animals. The cow has a long-term immunological memory in the protective system of the mammary glands which resonates depending on the production processes, and with its help the cows pass their immunological experience to their offspring (Chamorro et al., 2007, Kelling and Topliff, 2013, Munoz-Zanzi et al., 2003).
Immunization of dams is successfully used for preventive purposes (Silva et al., 2018). However, there are many cases of infectious diseases in bovine offspring born to vaccinated animals which is indicative of a low effectiveness of vaccination (Lanyon et al., 2014, Meganck et al., 2015).
Scientists from Brazil studied the effectiveness of vaccination against colibacteriosis at the probiotic therapy background. Each animal was vaccinated with two doses of the K99 and A14 Escherichia coli vaccine and a probiotic containing Lactobacillus acidophilus. The results showed that the administration of the vaccine together with a probiotic during 15 and 30 days was most effective for the specific prevention of infectious diarrhea (Avila et al., 1995).
With the purpose of increasing the effectiveness of vaccination and reducing post-vaccination complications, drugs that affect the functions of the immune system and stimulate the development of an immune response are widely used in veterinary medicine (Valpotić et al., 1985). Currently, there is a large range of immunostimulating drugs with different mechanisms of action on the market that are used to increase the effectiveness of vaccines (Renaud et al., 2019; Tagirov et al., 2018; Valpotić et al., 1986).
In connection with the above, the aim of the research was to correct colostral immunity using the immunostimulator “Immunat” in connection with vaccination for improving the safety of young animals and increasing body weight gain.
Materials and Methods
Research and production experiments were carried out in a livestock farm of the Republic of Bashkortostan. The farm has 350 seedstock herd animals, and 80 calves under the age of one month.
20 pregnant cows of black-and-white breed, as well as calves born to them from birth to two months of age were the object of research.
Animals for the research were selected on the basis of analogues and were in the identical conditions of feeding and maintenance.
The work used:
1. “Combovac”: – inactivated combined vaccine against infectious rhinotracheitis, parainfluenza-3, viral diarrhea, BRSV, rota- and coronavirus diseases of calves (scientific development and production centre “Narvak”, Moscow).
2. “Immunat” - a drug produced in Belarus ( BelAgroGen Research and Production Centre). The sodium salt of ribonucleic acid induces a leukocyte reaction, stimulates hematopoiesis, natural resistance (phagocytic activity, increases the content of lysozyme, properdin, β-lysine, the level of interferon synthesis), T - and B - immune systems, increases the antitoxic resistance of the animal body, accelerates tissue regeneration processes.
Pregnant vaccinated cows that did not receive the immunostimulator were the control (n=10) group. Vaccination was performed twice according to the instruction: for the first time, the cows were injected into the cervical region subcutaneously at a dose of 2 ml 40 days before calving, for the second time –20 days before calving.
Animals of the second (experimental) group (n=10) were vaccinated in the same way. 48 hours before vaccination, the immunostimulator “Immunat” was administered at a dose of 5 ml per animal.
Newborn calves were divided into four groups according to the division of their mother cows in the first experiment, of 5 heads in each. Calves from each group of cows were divided into 2 groups. In the first group of calves born to cows of the first (control) group, the drugs were not used, which was the control for the second stage of the research. Also, the immunostimulating drug was not applied in the third (experimental) group of calves born to mother cows of the second (experimental) group. Calves of the second and the fourth (experimental) groups were immunized with the drug “Immunat” at a dose of 2 ml per animal. The newborn calves were vaccinated against associative infections with Combovac at the age of 30 days twice with an interval of 20 days at a dose of 1 ml into the cervical region subcutaneously.
Serological tests of blood serum samples for the presence of anti-rotavirus, anti-diarrhea virus, and anti-coronavirus antibodies prior to vaccination, 40 days before calving, 20 days before calving, before calving, and in the colostrum of the first milk yield were performed in mother cows.
To assess colostral immunity in calves born to vaccinated cows, serological studies of blood serum sampled before colostrum intake, one day after its first intake, as well as on the 7th, 14th, 21st days of the calf’s life and at the age of 30 and 60 days were performed. Blood serum and colostrum samples from control and experimental animals were tested for the presence of antibodies to rotavirus and viral diarrhea in the immunoenzymatic assay (ELISA test), and to coronavirus in the hemagglutination-inhibition reaction (HI test).
Enzyme immunoassay. The reaction was performed with the use of a flat-bottomed polystyrene plate by two-fold serum dilution. One day before the reaction rotavirus antigen was introduced into all the wells of the plate. Samples of native sera were diluted with PBS buffer (phosphate-buffered saline) in a ratio of 1:50. Then, after washing with buffer, 0.1 ml of PBS buffer w/Twin was added to all wells of the plate. After that, 0.1 ml of test sera and the same amounts of negative serum and immunoglobilin were added to the first wells of each long row as negative and positive controls. Titrating was carried out by getting consistent, two-fold dilutions. The titrated serums were placed in the thermostat for 1.5 hours, then washed four times with a twin buffer. After that, 0.1 ml of the working anti-species conjugate was added to each well and placed again in the thermostat for 1.5 hours. Then the plate was washed with a twin buffer 4-5 times and 0.1 ml of a preprocessed substrate mixture of 5 - aminosalicylic acid with hydrogen peroxide was added to each well. The reaction was assessed visually 40-60 minutes later taking into account the color of the reaction product, which varies from dark to light brown. In the negative control, the solution remained transparent.
Hemagglutination inhibition consumption test. The reaction was performed in wells with a U-shaped bottom of a plastic panel by double dilution of sera with PBS buffer. After that, equal amounts of working dilution (4 AE) of the coronavirus antigen were added to all panel wells. After an hour of serum-antigen contact, a 0.5% suspension of white mice red blood cells was added to all panel wells. The reaction was assessed 60 minutes later. The highest amount of serum dilution, which inhibited the agglutination of red blood cells with coronavirus antigen by at least ++, was taken as the serum titer. The reaction control was the interaction of specific and control sera with the coronavirus antigen, for which the sera were titrated as field sera. The serum was considered positive if its titer was 1:32 or higher.
Statistical Analysis
Quantitative determination of the concentration of immunoglobulins A, M, and G in the tested animal sera was performed using the immunochemical automatic analyzer “Real Best”.
Statistical processing of the experimental data was performed using the statistical analysis package for Microsoft Excel®. The reliability of differences between the groups was evaluated using the Student t-test from p≤0.05 to ≤0.001.
Results and Discussion
The scientific novelty of our research lies in the fact that for the first-time complex studies of humoral factors of innate immunity and immunological reactivity, live weight gain, and the safety of newborn calves when using the immunostimulating drug “Immunat” related to vaccination were conducted.
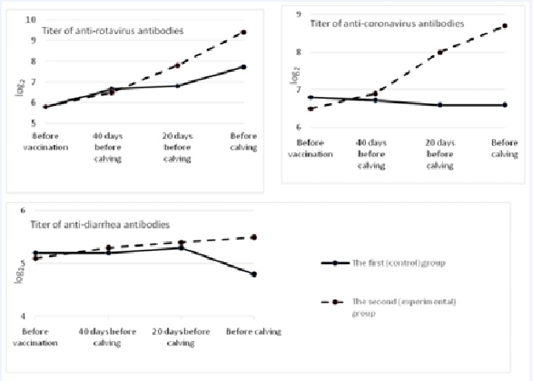
In the blood sera of pregnant cows after vaccination, there was a significant increase in the titers of anti-rotavirus antibodies in comparison with the prechallenge ones (before vaccination). The prechallenge antibody titer (before vaccination) was 5.8 1og2. By the time of calving, it increased in the experimental group by 3.62 1og2 and amounted to 9.4±0.34 (p<0.01). In the control group, antibody titers in cows before calving equaled 7.72±0.25 log2 (Figure 1).
In the first (control) group, the level of antibodies to coronavirus practically did not change during the experiment, and by the time of calving it slightly decreased and came to 6.6±0.25 log2. In the second (experimental) group, the increase in post-vaccinal antibodies in cows was the highest. It exceeded the background value by 2.2 1og2 and amounted to 8.7±0.26 1og2 (p<0.01). The difference between antibody titers before calving in the control and experimental groups made 2.1 log2 (p<0.01).
Before vaccination, the titer of antibodies to the causative agent of viral diarrhea in both groups of cows was at the level from 5.1 to 5.2 log2. In the control group, after vaccination and revaccination, that indicator did not change much, and by the time of calving it decreased to 4.8±0.21 log2. In the group of cows in which “Immunat” was used, the titers of humoral antibodies after immunization of pregnant cows increased by 0.4 1og2 in comparison with the prechallenge ones and amounted to 5.5±0.1 1og2 by the time of parturition. The difference between antibody titers before calving between the control and experimental groups was 0.7 1og2 (p<0.05).
Figure 2 shows the dynamics of colostrum immunoglobulins in the first hours after calving and over the course of seven days. When analyzing the data, a general tendency for the reduction of the concentration of immunoglobulins in the colostrum of cows in the control and experimental groups was observed. Primary concentration of immunoglobulins in the colostrum of cows of both groups was at the level from 89.6±3.8 g/l to 91.4±5.3 g/l. Within 12 hours, there was a sharp decrease in the content of immunoglobulins in the colostrum of cows which was a physiologically normal event (Ferdowsi Nia et al., 2010). However, it should be noted that the decrease in the level of immunoglobulins in the experimental group was less than in the colostrum of cows in the control group. The trend was observed throughout the research period. For example, 24 hours after calving, the difference in the content of immunoglobulins in the colostrum of cows of the control and experimental groups equaled 6.1 g/l (p<0.01). On the seventh day after calving, the same difference was 6.9 g/l (p<0.01). Thus, the level of immunoglobulins in the colostrum of cows that were treated with the drug “Immunat” was higher during the trial period.
In their studies, Usachev and Polyakov (2007) concluded that to increase the life stability of newborn calves, it is necessary to feed them bovine colostrum, rich in immunoglobulins.
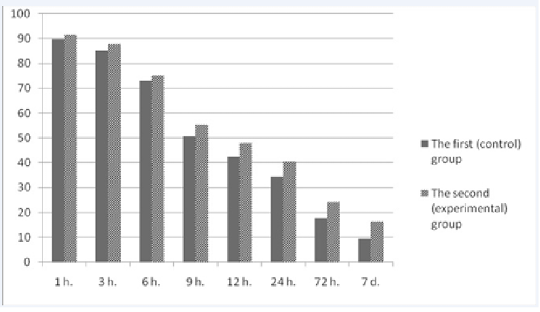
Figure 2: Changes in the content of immunoglobulins in bovine colostrum during the first week after calving
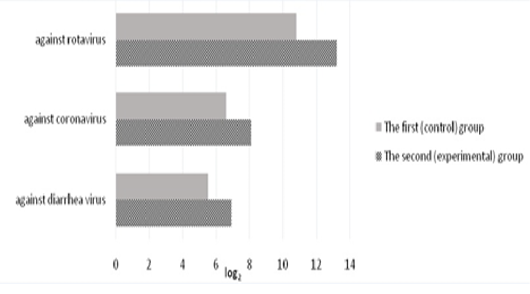
The level of specific antibodies to rotavirus, coronavirus, and diarrhea virus in the colostrum of the first milk yield is shown in Figure 3. In the colostrum of the first milk yield in calved cows of the control group, the titers of anti-rotavirus antibodies were 10.8±0.31 log2. In the experimental group, the titers of colostrum antibodies in experimental cows were 13.2±0.29 log2 which exceeded the control values by 2.4 log2 (p<0.01).
Antibodies to coronavirus in the colostrum on the day of calving were present in all experimental animals. On the second day, the level of antibodies decreased by 2-3 times, and only a small amount of them was found in the milk of the third day of lactation in 15-20% of animals. In the experimental group of animals, the titers of anti-coronavirus antibodies on the day of calving were 8.1±0.17 1og2 exceeding the control by 1.5 1og2 (p<0.01).
The titers of secretory colostrum antibodies against diarrhea virus of the calved cows of the first (control) group were 5.5±0.18 log2. In the experimental group, the titers of secretory colostrum antibodies in the first milk yield were 6.9±0.22 log2 and exceeded the control values by 1.4 log2 (p<0.01).
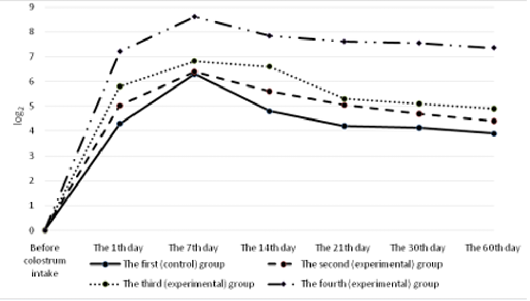
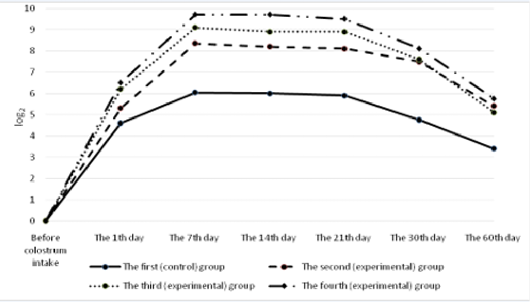
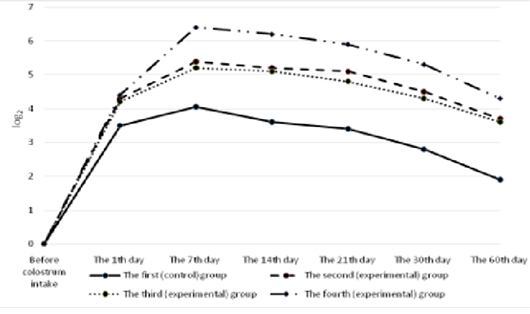
All the calves had no antibodies to rotavirus in their blood serum before colostrum intake (Figure 4). A day after the first colostrum reception, the antibody level in newborn calves was in the range of 4.3±0.25 to 8.1±0.33 1og2. Analyzing the data obtained for all groups, it can be noted that by the end of the first week of life of animals, antibodies to rotavirus reached the maximum values, and a month later there was a slight decrease in all groups of calves. Then, by the age of two months, the titer of humoral antibodies increased in the experimental groups and decreased in the control groups of animals. It was also natural that in all cases the values of experimental indicators of antibody titers against rotavirus significantly exceeded the control ones. In these terms, the maximum difference between the experimental and the control groups was registered in calves of the fourth experimental group.
All the calves had no antibodies to coronavirus in their blood serum before colostrum intake (Figure 5). Serum antibody titers reached their maximum values by the end of the first week of animal’s life, the highest level was observed in calves of the fourth group. By the end of the study period (60 days), a gradual decrease was observed making 5.6±0.6 1og2 in the experimental group and 3.4±0.4 1og2 (p<0.01) in the control group.
The calves of the control and experimental groups had no antibodies to diarrhea virus before colostrum intake (Figure 6). A day after colostrum reception, the antibody content in experimental animals was at the level from 4.2±0.19 log2 to 4.4±0.26 log2 which slightly exceeded the indicators of calves in the control group. By the end of the first week of life, the titer of humoral antibodies in the experimental groups of calves reached the maximum values. The highest deviations from the values of the control group were registered in calves that were immunized with the immunostimulator after birth and that were born to dams immunostimulated before vaccination during pregnancy. In the fourth (experimental) group, the indicator was maximum and exceeded the control by 2.35 log2 (p<0.01). Subsequently, the antibody titers of all the animals studied gradually decreased but the indicators of the experimental groups consistently exceeded the data of the control group.
Iranian scientists (Al-Alo et al., 2018) found that the level of colostral antibodies was the main indicator of passive immunity and protection against diarrhea in newborn calves.
In their studies Hodgins and Shewen (2012) found that vaccination of animals during pregnancy did not guarantee the accumulation of a sufficient number of specific antibodies in newborn animals. Danish scientists Andersen et al. (2017) established the effect of immunostimulating drugs (based on interferon) on the increase in antibody titer in farm birds.
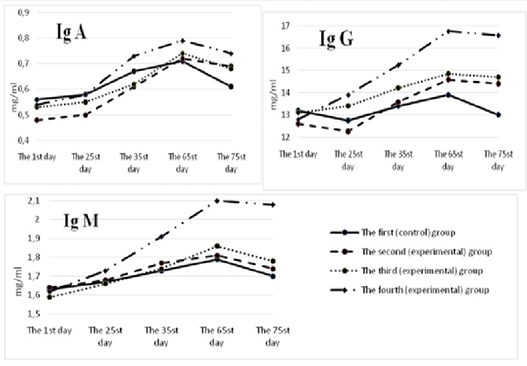
The dynamics of immunoglobulins was monitored for 75 days (Figure 7). At the beginning of the study, immunoglobulin A in the serum of calves of the control and experimental groups was at the level of 0.45±0.013 – 0.57±0.008 mg/ml, immunoglobulin M –1.57±0.003 – 1.64±0.029 mg/ml, immunoglobulin G –12.6±0.21-13.2±0.12 mg/ml.
For all the periods of the study, the content of immunoglobulins A, M, and G in the blood serum of calves of the control group was lower than the similar indicators of the experimental groups. The maximum difference between those indicators and the control was found in the animals of the fourth group. So, on the 65th day of the experiment in the fourth group, the content of immunoglobulins A, M, G was higher than in the control group by 0.08; 0.31 (р<0.05); and 2.86 (р<0.05) mg/ml, respectively. However, on the 75th day of the experiment, there was a decrease in the number of serum immunoglobulins A, M, and G in the studied groups of calves. The maximum decrease was registered in the control group – by 0.04; 0.09 and 0.9 mg/ml, respectively. In the fourth group of calves, the smallest decrease in immunoglobulins A, M, G was observed: by 0.01; 0.02 (р<0.05) and 0.18 mg/ml.
Calves of all experimental groups at the age of 30 days outnumbered their peers in the control group in terms of live weight. Thus, the live weight of calves of the second, third and fourth groups was greater than the weight of calves of the control group by 1.3; 3.9 and 5.7 kg, respectively.
The difference in the average daily live gain between the first (control) and second, third and fourth groups was 44.9; 81.5 and 158.1 g, respectively.
It should also be noted that by the age of 30 days in the second, third and fourth groups, the absolute weight gain of calves exceeded the control indicators by 1.1; 1.19 and 1.36 times, respectively.
Thus, the calves born to cows that were challenged with the immunostimulating drug “Immunat”, as well as newborn animals themselves, immunostimulated with the same drug, had significant differences in the absolute, average daily gain in live weight compared to control animals.
In the control group of animals, during the first three weeks of life, three calves were diagnosed with a disease that developed, as a rule, in an acute form, expressed by diarrhea with the release of fetid feces with a large amount of mucus, sometimes blood and fibrin, general depression, decreased appetite, a slight or moderate increase in body temperature and cachexy. Of the sick, two calves died. In the pathological material taken from the calves carcasses, laboratory tests (ELISA) identified rota- and coronavirus antigens, which confirms the involvement of these pathogens in the etiology of the disease.
During the first two weeks of monitoring in the experimental groups, in two cases, the animals had a clinical manifestation of diarrhea. Their illness was mild, lasted no longer than two days, and ended in complete recovery. There were no other cases of morbidity or mortality in the experimental groups.
A number of scientists (Pestka, 2007) conducted research on farm animals for the prevention and treatment of viral and bacterial infections using interferons and interferogens and proved their positive effect on the incidence rate. By the research of Ridpath et al. (2003) it was found that feeding newborn calves with the colostrum of cows that were challenged with immunostimulating drugs contributed to the live weight gain.
Our research has shown that the immunostimulator “Immunat”, having an immunocorrecting effect, activates the formation of antibodies to vaccine antigens for the prevention of associative infections of calves, as a result of which it has a positive effect on improving the growth and safety of newborn calves.
Thus, it has been established that:
challenging pregnant cows with the drug “Immunat” (48 hours before immunization) at a dose of 5 ml per animal increases the amount of specific antibodies to rota-, coronavirus, and diarrhea virus in the serum prior to calving by 1.68; 1.68 m; 0,7 log2, respectively; raises the content of immunoglobulins by 1.8 g/l and increases the number of antibodies to rota-, coronavirus, diarrhea virus in colostrum of the first milk yield by 2,4; 1,5; 1,4 log2, respectively, compared to the same indicators of cows that were not challenged with the drug; feeding newborn calves with the colostrum of dams which contains specific antibodies to rota-, coronavirus and diarrhea virus, and administration of immunostimulant “Immunat” at a dose of 2 ml per animal in the first days of life induces the increase in the titer of anti-rotavirus antibodies by 2.3 log2; of anti- coronavirus – by 3.66 log2; of anti-diarrhea virus – by 2, 35 log2, respectively, and, as a consequence, contributes to the prevention of morbidity and mortality, and also increases the main indicators of growth and development of calves.
acknowledgements
The authors are thankful to Federal State Budgetary Educational Institution of Higher Professional Education “Bashkir State Agrarian University” for providing necessary facilities for the research. The authors did not receive any fund for this study.
Conflict of interest
Authors declare that they have no conflict of interests.
authors contribution
AA designed the study, wrote and revised the manuscript, and managed correspondence. ON applied testing. OA collected the results of this test. CG and RA collected the results of this test and wrote the manuscript. All authors read and approved the final manuscript.
References