Advances in Animal and Veterinary Sciences
Research Article
Efficacy of Green Tea Extract in Tributyltin Induced Toxicity on the Testes of Adult Albino Rats
Marwa G. Ahmed1*, Mona El-Demerdash Ibrahim1, Hoda Ragab El-Sayed1, Samah M. Ahmed2
1Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig, Egypt; 2Department of Histology and Cell Biology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Abstract | Male infertility is one of the challenging problems encountered by human society worldwide. Several reports suggest a likely association between deteriorating reproductive male health and exposure to endocrine disruptors (ED). Tributyltin (TBT), a well-known ED, is an organotin compound that is highly toxic and widely used in industry, agriculture, and antifouling paints. This study aimed to assess the toxic impact of tributyltin on the testes of adult male albino rats and the possible protective role of green tea extract. Forty-two adult male albino rats were divided into five groups; Group I (control group), Group II (green tea treated group) each rat received green tea extract (150 mg/kg body weight) dissolved in 1 ml distilled water orally for 8 weeks, Group III (TBT treated group) each rat received the 1/20 of LD50 of tributyltin chloride (5 mg/kg/day) dissolved in1/2 ml corn oil orally for 8 weeks; Group IV (TBTand green tea group) each rat received green tea extract in the previous dose then tributyltin chloride in the same dose orally for 8 weeks; Group V (Follow up group) each rat received tributyltin chloride in the same dose for 8 weeks, then tributyltin chloride was stopped and rats were examined four weeks after its discontinuation. At the end of the study, animals were subjected to biochemical, histological, immunohistochemical, and seminal examination. The results revealed a statistically significant decrease in testosterone, FSH, LH, and serum glutathione peroxidase levels and a significant increase in serum Malondialdehyde in the TBT group when compared with the control group. Rats treated with tributyltin showed severe histopathological changes and decreased cell proliferation in the testes. Seminal analysis of the TBT group showed a significant affection of all parameters when compared to other groups. The follow-up group still showed the persistence of all changes. Green tea was found to ameliorate all of these hazardous effects nearly to the control values. In Conclusion, TBT induces oxidative stress and hormonal disturbance, leading to testicular degeneration. Green tea extract has a beneficial role in ameliorating TBT induced reproductive toxicity.
Keywords | Tributyltin, Infertility, Testes, Oxidative stress, Green tea
Received | June 21, 2020; Accepted | September 16, 2020; Published | December 03, 2020
*Correspondence | Marwa Gabr Ahmed, Faculty of Medicine, Department of Forensic Medicine and Clinical Toxicology, Zagazig University, Zagazig 44519, Egypt; Email: marwagabr2188@gmail.com
Citation | Ahmed MG, Ibrahim ME-D, El-Sayed HR, Ahmed SM (2021). Efficacy of green tea extract in tributyltin induced toxicity on the testes of adult albino rats. Adv. Anim. Vet. Sci. 9(1): 1-14.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.1.14
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Infertility is a growing concern and a significant health problem affecting human life worldwide (Mitra et al., 2017). Several factors contribute to male infertility. The most important is the exposure to environmental contaminants as endocrine disruptors (Mahmoud et al., 2019). Tributyltin is a worldwide environmental pollutant. It belongs to the class of organotins which are diverse groups of widely distributed, highly toxic chemicals that have been implicated as endocrine disruptors (Barbosa et al., 2018). The widespread use of TBT as a biocide in antifouling paints and agriculture fungicide on food crops has led to environmental and marine pollution (Santos-Silva et al., 2018). Human exposure occurs mainly through the ingestion of TBT contaminated seafood, drinking contaminated water, skin contact, and inhalation in the vulnerable population (Dogan et al., 2017).
Tributyltin is an ED in mammals, leading to imposex (a superimposition of male features in females) in female gastropod mollusks (Santos-Silva et al., 2018). Tributyltin and its organotin metabolites (such as dibutyltin and inorganic tin) accumulate in the tissues of various experimental animals due to its lipophilic property causing liver, nervous, and immune system disorders (Dogan et al., 2017). Most of the studies that investigated the mechanisms of tributyltin toxicity have suggested both oxidative stress and apoptosis (Mitra et al., 2014).
Green tea is a beverage utilized globally. It has captured considerable attention for its health benefits against a variety of toxins associated with oxidative stress (Idowu, 2017). Most of its significant effects are attributed to its polyphenolic flavonoids, known as catechins. Green tea extract (GTE) contains also additional antioxidants such as vitamins E and C, as well as minerals that function as co-factors for antioxidant enzymes (Ibrahim et al., 2015). The most pervasive recognized properties of green tea are their antioxidant activities, arising from their ability to scavenge reactive oxygen species (ROS) in addition to their chelating properties (El-Dine et al., 2017). So, the present work aimed to evaluate the effect of TBT on the testes of adult albino rats and the possible protective role of green tea extract through biochemical, histological, immunohistochemical, and seminal examinations.
MATERIALS AND METHODS
Chemicals
Animals and experimental design
The present study was carried out on 42 adult male albino rats, each weighing 150-200 gm with an average age of 50-60 days. They were obtained from the animal house, Faculty of Medicine, Zagazig University, Egypt.
The rats received balanced food rich in all stuff necessary to maintain their health before and during drug administration. It consisted of bread, barley, and milk. Water was offered in separate clean containers. All animals received humane care according to the animal care guidelines of the National Institutes of Health (NIH), and the local committee approved the design of the experiments.
Grouping of animals
The rats were divided into five groups:
Group I (control group) (18 rats): was further divided into 3 equal groups
Group IA: (negative control group): Rats received only regular diet and tap water for 8 weeks to measure the basic parameters.
Group IB (positive control: Corn oil group): Rats were treated with 1/2 ml corn oil daily by oral gavage for 8 weeks.
Group IC (positive control: Distilled water group): Rats were treated with 1 ml distilled water daily by oral gavage for 8 weeks.
Group II (Green tea group) (6 rats)
Rats of this group received green tea extract of 150 mg/kg body weight dissolved in 1 ml of distilled water by oral gavage for 8 weeks.
Group III (TBT -treated group) (6 rats)
Each rat in this group received Tributyltin chloride in a dose of 5 mg/kg/day (which represents 1/20 of LD50 of tributyltin chloride in rats) (Mitra et al., 2014) dissolved in 1/2 ml corn oil orally by gavage for 8 weeks.
Group IV (Tributyltin and green tea group) (6 rats)
Rats of this group received green tea extract (150 mg/kg body weight) (El dine et al., 2017) dissolved in 1 ml distilled water then 5mg/kg tributyltin chloride dissolved in 1/2 ml corn oil orally by gavage for 8 weeks.
Group V (follow up group) (6 rats)
Rats in this group received tributyltin chloride dissolved in corn oil in a dose of 5 mg/kg for 8 weeks then TBT was stopped. Rats were examined 4 weeks after its discontinuation.
At the end of the study ( After 8 weeks of treatment and 4 weeks follow up), rats of all groups were weighed then anesthetized using ether anesthesia, blood was collected from retro-orbital plexus for the determination of hormonal assay (testosterone, FSH and LH levels) and oxidative stress markers (glutathione peroxidase and malondialdehyde) in the serum. Rats were sacrificed, then the testes were weighed, semen was collected for analysis. Testes were subjected to histopathological examination using H and E stained sections, immunohistochemical staining for detection of Ki-67 as a marker of proliferation.
Assessment of serum testosterone, FSH, LH levels
Blood was collected from the retro-orbital plexus, centrifugation was done for 15 minutes and serum was kept at -20°C for analysis of hormones by ELISA, testosterone by Zirkin and Chen (2000), FSH, and LH by Kaya et al. (2013).
Antioxidant enzymes and lipid peroxidation
For assessment of oxidative stress markers, blood samples were collected into a clean centrifuge tube and incubated at 37°C until blood clotted and then centrifuged for 10 minutes to separate the serum, and a pipette was used to separate the top layer.
Assessment of serum malondialdehyde (MDA) level (nmol/ml)
Serum MDA was assayed using MDA biodiagnostic kit according to the method described by Draper and Hadley (1990) where MDA can react with Thiobarbituric Acid (TBA) and give pink-colored trimethine complex. Serum Malondialdehyde was determined from the absorbance on a spectrophotometer at 530-532 nm.
Assessment of serum Glutathione peroxidase activity (GPX) (ng/ml)
The GPX activity was measured using the GPX biodiagnostic kit according to the method of Paglia and Valentine (1967). The principle is based on the enzymatic reaction involving B-nicotinamide adenine dinucleotide phosphate (B-NADPH, reduced form), Glutathione peroxidase, and a sample or a standard which was initiated by addition of hydrogen peroxide. The change in the absorbance was monitored spectrophotometrically at 430 nm. A standard curve was plotted for each assay.
Methods used for epididymal spermatozoal examination
Spermatozoa collection was done as described by Kuriyama et al. (2005). The epididymal component of each rat was attained directly by cutting the tail of the epididymis and embracing it gently to obtain the fresh undiluted semen in a clean Petri dish and incubated at 37ºC for half an hour for liquefaction to continue to the subsequent examinations:
Sperm count: Estimation of the concentration of sperms was done by multiplying the counted number of sperms by 100 (depth) and 1000 (dilution) (Blazak et al., 1993; Assayed et al., 2008).
Progressive motility: The sperms’ progressive motility was estimated according to the method reported by Bearden and Flyquary (1980).
Sperm viability: Two hundred sperms were counted per rat under high power lens of light microscope and the number of live (unstained) and dead (stained) sperms were estimated among the two hundred sperms, then the percentage of viability was calculated according to the method reported by Björndahl et al. (2003).
Sperm abnormalities: A drop of epididymal content of each rat was mixed with an equal drop of eosin-nigrosin stain. The semen was carefully mixed with the stain, these films were spread on clean and grease-free slides. Two hundred sperms were randomly observed per rat under the high power lens of a light microscope and the percentage of abnormal sperms was recorded (Brown et al., 1994).
The description of the sperm abnormal forms observed in this study was done according to Mori’s classification (Mori et al., 1991) who classified sperm abnormal forms into tailless sperm, sperms with abnormal heads and sperms with deformed tails.
Methods used for histopathological examination of the testes
At the time of sacrifice, all animals were weighed then anesthetized by ether inhalation, then were sacrificed. The testes and epididymis were immediately dissected out, grossly inspected to assess any gross abnormalities and testicular weight was recorded. Both testes were fixed in Bouin’s solution, After fixation, testes were embedded in paraffin blocks and processed for the preparation of 5 μ thickness sections. These sections were stained with Hematoxylin and Eosin stain and examined by a light microscope (Bancroft and Layton, 2013).
Methods used for Immunohistochemical examination of the testes
Detection of Ki-67 antibodies was carried out using a streptavidin-biotin complex immunoperoxidase system. Paraffin-embedded sections of specimens were dewaxed and incubated in 0.1% hydrogen peroxide for 30 min to block the endogenous peroxidase followed by rinsing three times in phosphate-buffered saline (PBS). Antigen retrieval was achieved by microwave treatment (20 min, 0.01 Mol/L citrate buffer, pH 6). After demasking, the slides were incubated overnight with the primary antibody. Sections were incubated with rabbit monoclonal Ki-67 Antibody (SP6) (Cat. No. MA5- 14520, Thermo Fisher Scientific, Rockford, USA), diluted 1:200 and applied to sections for 12 h at 4 C. After several washes with PBS, primary antibodies were detected by incubation with biotinylated anti-rabbit antibodies (versal kits, Zymed Laboratories) for 30 min at room temperature (Ramos-Vara et al., 2008).
Histo-morphometric analysis
Analyzing images by computer system Leica Qwin 500 (Leica Ltd, Cambridge, UK) at the Image analyzing unit of Pathology Department, Faculty of Dentistry, Cairo University, was used to evaluate the area percent of Ki67 immune-reaction, the perimeter of the seminiferous tubules, and the epithelial height of germinal epithelium. The measurements were done by a menu of interactive measures. The measuring frame of a standard area equal to 118476.6 mm² was chosen. Ten readings from five non-overlapping sections from each rat of all groups were observed.
Statistical analysis
Analysis of data was done using the SPSS program (Statistical Package for Social Science) version 25.0. For normally distributed data, a comparison between the five studied groups was analyzed using a one-way analysis of variance (ANOVA or F-test) and the least significant difference (LSD) test. All data were expressed as mean ± SD (Standard deviation). A p-value of less than 0.05 was considered statistically significant (George and Mallery, 2018).
Results and Discussion
Regarding the control groups (negative control group, corn oil group, distilled water group), and green tea group
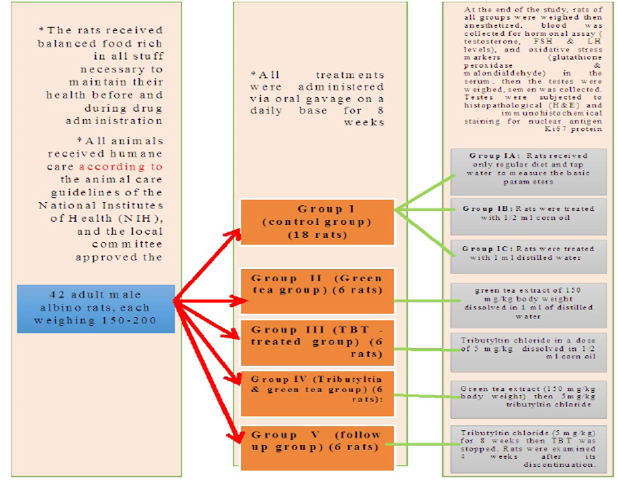
Control groups and green tea treated group showed no significant difference regarding the following parameters: the body weight, absolute or relative testicular weight, hormonal levels or oxidative stress parameters, sperm parameters, and histomorphometric parameters. Also, there were no histopathological changes in the testes of these groups So, we considered the -ve control group as the control one for comparison with other groups (Figures 1, 2, 3 and 10).
Regarding the results of the treated groups (TBT treated group, TBT and green tea group and follow up group)
Body weight and absolute and relative testicular weight
Regarding the body weight, there was an increase in the body weight in the TBT group and follow up group as compared to other groups but with no statistically significant difference (P> 0.05). No statistically significant difference between the control group and TBTand green tea group. Regarding absolute and relative testicular weight, there was a highly statistically significant decrease in the TBT group as compared to the control group (P < 0.001). A highly statistically significant increase of both testicular and relative testicular weight was observed in TBTand green tea group as compared to the TBT group (P < 0.001). There was a non-significant difference between TBTand green tea group and the control group (P > 0.05). There was a significant decrease in the follow-up group when compared to TBTand green tea and the control groups (P<0.05) (Figure 4).
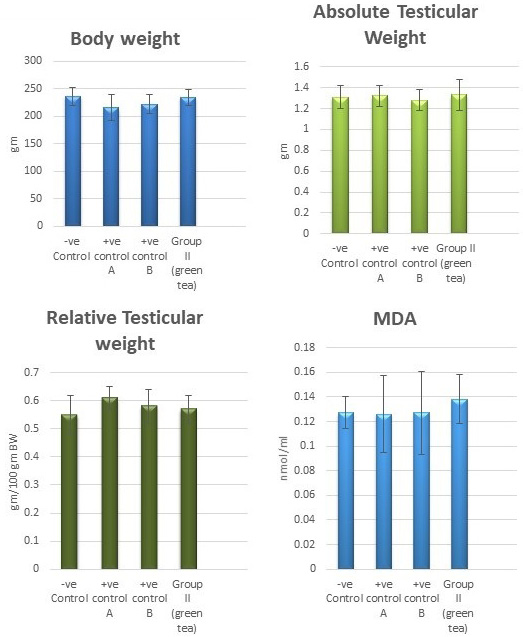
Figure 1: Bar charts showing the comparative magnitude of mean values of the body weight, testicular weight, relative testicular weight and MDA among the negative control group, positive control A (Corn oil group), positive control B (Distilled water group) and Green tea group (II).
Hormonal levels: Serum testosterone hormone, FSH, and LH hormone levels
Regarding testosterone, FSH and LH hormonal levels among the studied groups, there was a highly statistically significant decrease in the mean hormonal levels in TBT group as compared to the control (P < 0.001) while there was a highly significant increase in TBTand green tea group when compared to TBT group (P < 0.001). There was a non-significant difference in TBTand green tea group when compared to the control group (P > 0.05). A significant decrease in the follow-up group when compared to other groups was noted (P<0.05) (Figure 5).
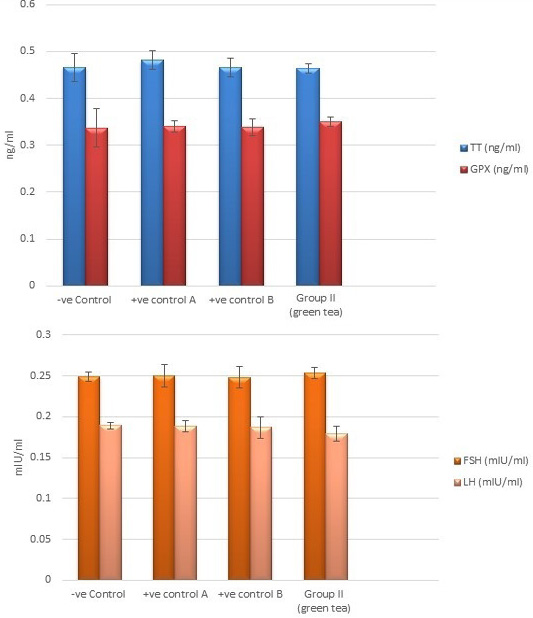
Figure 2: Bar charts showing the comparative magnitude of mean values of testosterone, GPX, FSH, and LH levels among the negative control group, positive control A (Corn oil group), positive control B (Distilled water group) and Green tea group (II).
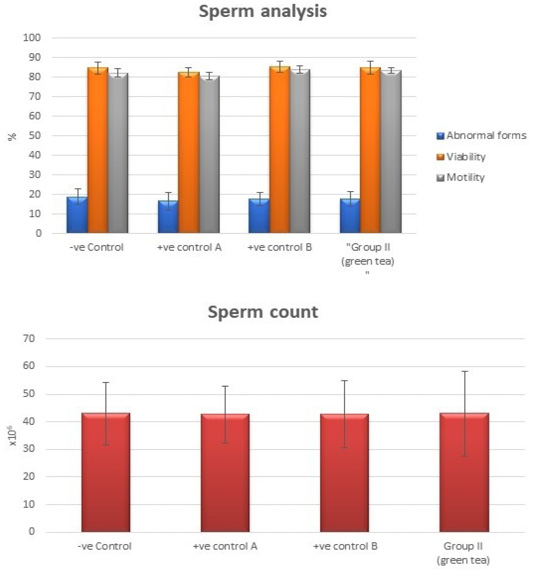
Figure 3: Bar charts showing the comparative magnitude of mean values of sperm count, abnormal forms, sperm motility, and sperm viability among negative control group, positive control A (Corn oil group), positive control B (Distilled water group) and Green tea group (II).
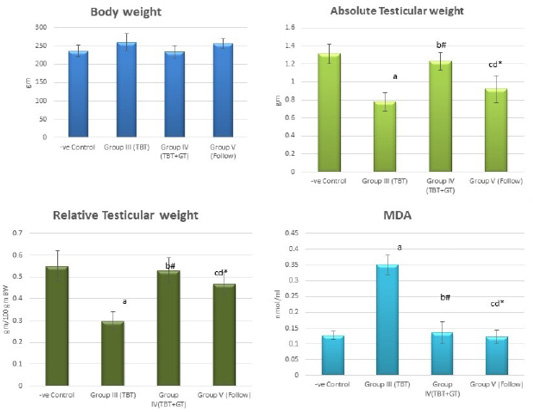
Figure 4: Bar charts showing the comparative magnitude of mean values of the body weight, absolute testicular weight, relative testicular weight and MDA among the negative control group (I), TBT treated group (III), TBT and green tea group (IV) and follow up group (V). #p>0.05, *p<0.05, ap<0.001 compared to control group, bp<0.001, cp<0.05 compared to TBT group, dp<0.05 compared to TBT +GT group. Values are presented as mean ± SD (n= 6 in each group).
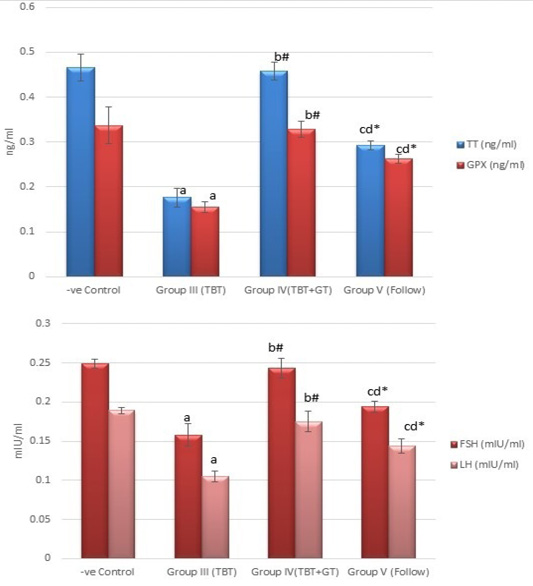
Figure 5: Bar charts showing the comparative magnitude of mean values of testosterone, GPX, FSH, and LH levels among the negative control group (I), TBT treated group (III), TBT and green tea group (IV) and follow up group (V). #p>0.05, *p<0.05, ap<0.001 compared to control group, bp<0.001, cp<0.05 compared to TBT group, dp<0.05 compared to TBT +GT group. Values are presented as mean ± SD (n= 6 in each group).
Oxidative stress parameters: Malondialdehyde (MDA) level (µmol/L) and Glutathione peroxidase (GPX) activity (ng/ml) in the serum
There was a highly significant increase in MDA levels in the TBT group when compared to the control group (P < 0.001) while there was a highly significant decrease in TBTand green tea group when compared to the TBT group (P < 0.001). There was a non-significant difference in MDA levels in TBTand green tea group as compared to the control group (P > 0.05) while there was a significant increase in follow up group when compared to other groups (P<0.05) (Figure 4).
As regards GPX levels, there was a highly statistically significant decrease in the TBT group as compared to the control (P < 0.001) while there was a highly significant increase in TBTand green tea group when compared to the TBT group (P < 0.001). There was a non-significant difference in TBTand green tea groups when compared to the control group (P > 0.05). A significant decrease in the follow-up group when compared to other groups (P<0.05) (Figure 5).
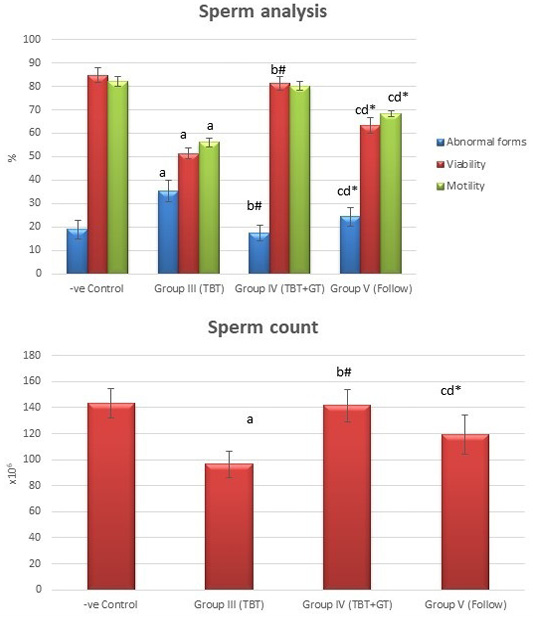
Figure 6: Bar charts showing the comparative magnitude of mean values of sperm count, abnormal forms, sperm motility, and sperm viability among the negative control group (I), TBT treated group (III), TBT and green tea group (IV) and follow up group (V). #p>0.05, *p<0.05, ap<0.001 compared to control group, bp<0.001, cp<0.05 compared to TBT group, dp<0.05 compared to TBT +GT group. Values are presented as mean ± SD (n= 6 in each group).
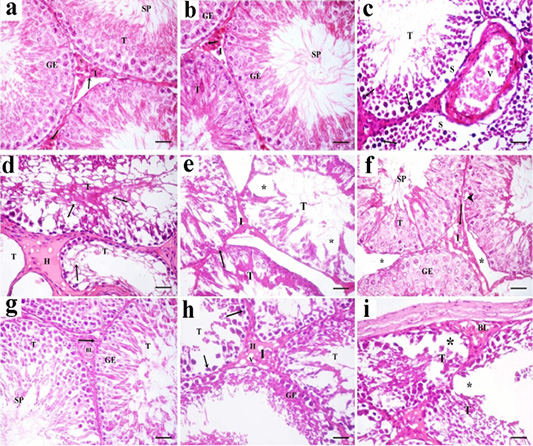
Figure 7: A photomicrograph of H and E stained sections of the control subgroups showing similar histological results, so the negative control group is considered as the control group. a: H and E stained sections of the control testes reveals numerous packed seminiferous tubules (T) that are lined by stratified germinal epithelium (GE). Spermatozoa (SP) are present in the lumen of seminiferous tubules. Interstitium (I) contains interstitial cells (arrow) and blood vessels (v). b: Examination of Hand E stained sections in rat testes of green tea group revealed also normal histological structure. Packed seminiferous tubules (T) that are lined by stratified germinal epithelium (GE). Spermatozoa (SP) are present in the lumen of seminiferous tubules. Interstitium (I) containing Leydig cells (arrow) is also seen; c: Sections in TBT treated group reveal marked disorganized seminiferous tubules (T). Some sections showing tubules with their germinal epithelium separated from their basal laminae (S). Germinal epithelial cells with dark pyknotic nuclei (arrow) are detected. Blood vessels (V) in the interstitium are dilated and congested; d: Other sections in TBT treated group showing seminiferous tubules (T) with a marked decrease in the epithelial height or lose their lining epithelium, other tubules had marked vacuolations (v). Acidophilic vacuolated hyaline material (H) is present in the interstitium in between the tubules; e: Sections in TBT treated group reveals shrinkage of the tubules (T) with irregular basement membrane (arrow), wide Interstitium (I), and areas of tissue loss in the lining germinal epithelium (*). f: Examination of sections in the TBT and green tea group showing improvement in the histological picture of the germinal epithelium (GE) of seminiferous tubules (T). Some spermatozoa (SP) are present in the lumen of the tubules. Separation between some tubules (*) and intracellular vacuolations (arrowhead) in some germinal epithelial cells are still detected; g: Other sections in TBT and green tea group showing normal histological structure. Tubules (T) are lined by germinal epithelium (GE). Spermatozoa (SP) are present in the lumen of the tubules. Interstitium (I) containing Leydig cells (arrow) and blood vessels (BL) is also detected; h: Sections in the follow-up group showing tubules (T) lined by germinal epithelial cells (GE), most of them had dark pyknotic nuclei (arrow). Interstitium (I) reveals hyaline (H) vacuolated material (V); i: Other sections in the follow-up group showing, disorganized tubules (T), congested blood vessels (BL), and marked cellular loss (*). H and E× 400 (scale bar 20µm).
Seminal analysis
Tributyltin treated group showed a highly statistically significant decrease in the mean values of sperm count, sperm motility and viability percent, and a highly significant increase in the sperm abnormal forms percent when compared to the negative control group (P < 0.001). In TBT and green tea group, a highly statistically significant increase in the mean values of sperm count, sperm motility, and viability percent and a highly statistically significant decrease in the mean values of sperm abnormal forms percent were noted when compared with TBT group (P < 0.001).While there was a non-significant difference in TBT and green tea group when compared to the control group (P > 0.05). There was a significant decrease in the follow-up group when compared to other groups (P<0.05) (Figure 6).
Figures 9a, 9b, 9c, 9d and 9e showed examples of the sperm abnormal forms detected in the TBT group as compared to the normal sperm forms.
Histopathological results
Sections of the control testes and green tea group showed numerous packed seminiferous tubules lined by germinal epithelium which had several types of cells; spermatogonia, primary spermatocytes, and spermatids. Spermatozoa were present in the lumen. Sertoli cells (supporting cells) had an important role in germ cell growth. In-between the seminiferous tubules, narrow interstitium containing interstitial cells and blood vessels was detected (Figure 7a and 7b ).
Sections in TBT treated group revealed marked disorganized seminiferous tubules and separation of germinal epithelium from basal laminae. Germinal epithelial cells with dark pyknotic nuclei were detected. Blood vessels in the interstitium were dilated and congested (Figure 7c). Other sections showed tubules that had lost their lining germinal epithelium, marked vacuolations, and disorganized epithelial lining. Acidophilic hyaline material was present in the interstitium in between seminiferous tubules (Figure 7d). Shrinkage of the tubules with irregular basement membrane, wide separation between them, marked decrease in the height of the lining germinal epithelium (Figure 7e).
Examination of sections in the TBT and green tea treated group showed improvement in the histological picture of the germinal epithelium of seminiferous tubules. Some spermatozoa were present in the lumen of the tubules. Separation between the tubules and intracellular vacuolations in some germinal epithelial cells were still detected. Other sections showed normal histological structure. Tubules were lined by germinal epithelium. Spermatozoa were present in the lumen of the tubules. Interstitium containing Leydig cells and blood vessels was also detected (Figures 7f and 7g).
Sections in the follow-up group showed tubules lined by germinal epithelial cells, most of them had dark pyknotic nuclei. Interstitium revealed hyaline vacuolated material. In other sections, disorganized tubules, congested blood vessels, and marked cellular loss were also detected (Figures 7h and 7i).
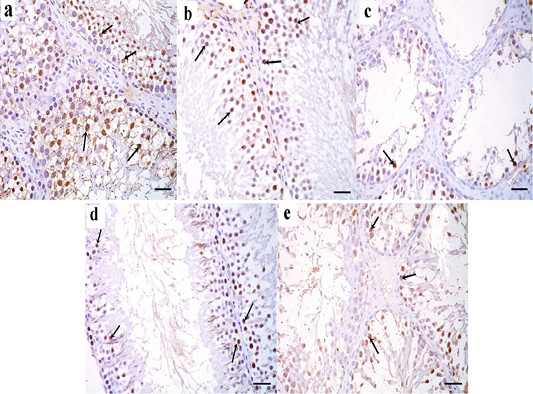
Figure 8: a: Immunohistochemical reaction for ki67 stained sections in the testicular tissue of the control group showing a strong positive reaction in the nuclei of germinal epithelial cells (arrow); b: Immunoperoxidase technique for ki67 in green tea treated group showing a strong positive reaction in the nuclei of germinal epithelial cells (arrow); c: In TBT treated group, a positive reaction for ki67 in the nuclei of few germinal epithelial cells is seen (arrow); d: TBT and green tea treated group reveals strong positive reaction in the nuclei of most of the germinal epithelial cells (arrow); e: In the follow-up group, a strong positive reaction is seen in the nuclei of some germinal epithelial cells (arrow). Immunoperoxidase technique× 400 (scale bar 20µm).
Immunohistochemical results
Immunohistochemically stained sections for ki67 protein in the testicular tissue of the control and green tea treated group showed a strong positive reaction in the nuclei of germinal epithelial cells (Figure 8a and 8b). In TBT treated group, a weak positive reaction for ki67 was seen in the nuclei of few germinal epithelial cells (Figure 8c). Tributyltin and green tea treated group showed strong positive reaction in the nuclei of most of the germinal epithelial cells (Figure 8d), however, in the follow-up group, a weak positive reaction was seen in the nuclei of some germinal epithelial cells (Figure 8e).
Sperm morphology
For each rat, 500 sperms were examined by using a hemocytometer for morphological abnormalities. In the control and green tea treated groups, normal sperm morphology was detected. Sperms had smooth heads with hooked tapering apex and normal long tails (Figure 9a and 9b). In TBT treated group, sperm abnormalities were detected in the heads and tails; curved sperms, headless tails, and abnormally shaped heads were seen (Figure 9c). In TBT and green tea treated group, marked improvement in sperm morphology as the sperms were with normal heads and tails (Figure 9d). In the follow-up group, some sperms still had sperm abnormalities such as headless tails and curved sperms (Figure 9e).
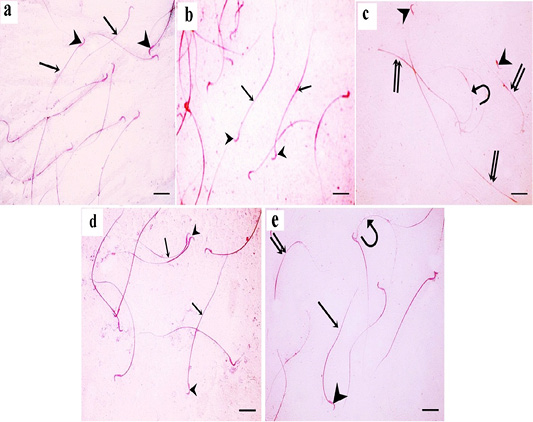
Figure 9: a: A photomicrograph of sperms from the control rats showing normal morphology with normal heads (arrowheads) and normal tails (arrows); b: In green tea treated group, normal sperm morphology is detected. Sperms have smooth heads with hooked tapering apex (arrowhead) and normal long tails (arrow); c: In TBT treated group, sperm abnormalities are detected in the heads and tails; curved sperms (curved arrow), headless tails (double arrow), and abnormally shaped heads (arrowhead) are seen; d: In TBT and green tea treated group, marked improvement in sperm morphology as the sperms are with normal heads (arrowhead) and tails (arrow). e: In the follow-up group, some sperms are with normal heads (arrowhead) and tails (arrow), however, others still have abnormalities such as headless tails (double arrow) and curved sperms (curved arrow). Eosin-nigrosin X 400 (scale bar 20µm).
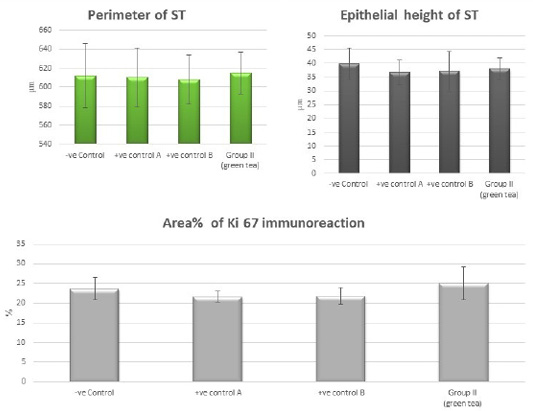
Figure 10: Bar charts showing the comparative magnitude of mean values of the perimeter of ST, the epithelial height of ST and area% of Ki 67 immunoreaction among the negative control group, positive control A (Corn oil group), positive control B (Distilled water group) and Green tea group (II).
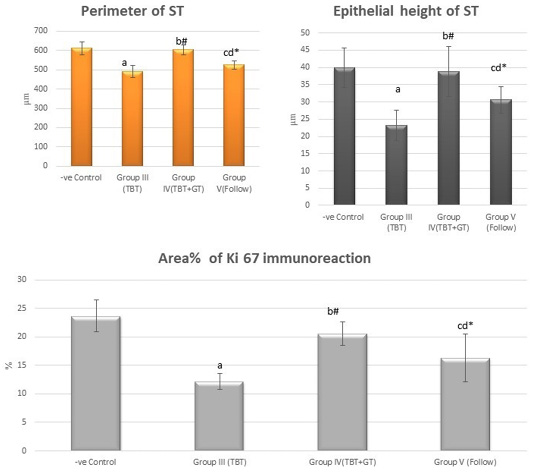
Figure 11: Bar charts showing the comparative magnitude of mean values of the perimeter of ST, the epithelial height of ST and area% of Ki 67 immunoreaction among the negative control group (I), TBT treated group (III), TBT and green tea group (IV) and follow up group (V). #p>0.05, *p<0.05, ap<0.001 compared to control group, bp<0.001, cp<0.05 compared to TBT group, dp<0.05 compared to TBT +GT group. Values are presented as mean ± SD (n= 6 in each group).
Histo-morphometric results
There was a highly statistically significant decrease in the perimeter and epithelial height of seminiferous tubules and area% of Ki 67 immunoreaction in the TBT treated group as compared to the control group while there was a highly significant increase in TBTand green tea group when compared to TBT group (P < 0.001). There was a non-significant difference in TBTand green tea group when compared to the control group (P > 0.05). There was a significant decrease in the follow-up group when compared to other groups (Figure 11).
The present study revealed an increase in body weight among the TBT group as compared to the control group with no statistically significant difference. Li et al. (2015) stated that tributyltin initiates adipocyte differentiation in vitro and increases its mass in vivo. Tributyltin chloride has been identified recently as an obesogen because it induces adipogenesis through signaling between retinoid X receptor (RXR) and peroxisome proliferator-activator receptor gamma (PPARγ) (Pivonello et al., 2020). The increase in body weight can also be explained by hormonal changes due to abnormal hypothalamic-pituitary function as agreed by Sena et al. (2017). Guo et al. (2018) mentioned that TBT exposure resulted in increased weight gain.
A highly significant decrease in the mean values of both testicular and relative testicular weight in TBT- treated group as compared to the control group was detected in the current study. Kanimozhi et al. (2014) reported that TBT caused a severe loss in the testicular weight. the significant reduction in testicular weight might be due to testicular hypocellularity, shrinkage, and atrophy of seminiferous tubules and spermatogenesis suppression which were noted in our study as Hajizadeh et al. (2016) reported that testicular weight largely depends on the population of differentiated spermatogenic cells.
Upon supplementation with green tea to TBT treated rats, the bodyweight returned to control values compared to the TBT group but with no statistically significant difference. Also, there was a highly significant increase in the mean values of both testicular and relative testicular weight in tributyltin and green tea group when compared with the TBT group. The protective effect of green tea can be explained by inhibiting oxidative damage and cellular apoptosis as concluded by Abdelrazek et al. (2016) who reported that administration of green tea in male Wistar rats significantly restored the reduction in body weight and significantly improved relative organs weight of testes, prostate, and seminal gland. Mosbah et al. (2015) showed that green tea extract protected against oxidative stress therefore reduced body growth and reproductive toxicity. Heikal et al. (2014) also mentioned that administration of green tea extract for 70 days significantly restored the decrease in sexual organs weights by attenuating testicular oxidative damage.
In the current work, TBT treated rats showed a highly significant decrease in the serum testosterone, FSH, and LH as compared to the control group. The low serum testosterone concentration may be linked to the inhibitory effect of TBT on the secretion of pituitary gonadotropins (FSH and LH) which are involved in testosterone biosynthesis as suggested by Merlo et al. (2016), Sena et al. (2017) and Santos-Silva et al. (2018) that TBT produces disruption of the hypothalamic-pituitary axis function. Another explanation is by direct inhibition of Leydig cells, or by inhibition of steroidogenesis in the testis as stated by Mitra et al. (2014).
Moreover, according to Wu et al. (2017), TBT blocked Leydig cell regeneration resulting in a severe reduction in serum testosterone levels. Furthermore, the increased oxidative stress in Leydig cells might be responsible as ROS can have detrimental effects on critical components of the steroidogenic pathway as reported by Mitra et al. (2014). Another theory for TBT induced reproductive toxicity is by being obesogenic since Pivonello et al. (2020) reported that obesity promotes male infertility through hormonal disruption, apoptotic germ cell death and altered redox status.
Co-administration of green tea with TBT resulted in a highly significant increase in serum testosterone, FSH, LH when compared with the tributyltin group. This protective effect may be related to the reduction of oxidative damage and improvement of testicular tissue integrity, which were observed in the present study due to its antioxidant, anti-inflammatory and anti-apoptotic effects as reported by El Dine et al. (2017), Akinwande et al. (2019) and Bagherpour et al. (2019). Fouad et al. (2017) reported that epigallocatechin-3-gallate (EGCG), the main active flavonoid in green tea, caused significant increases in serum testosterone and testicular protection in rats by its antioxidant and antiapoptotic effects.
In the present work, rats treated with tributyltin showed a highly significant decrease in serum GPX and a highly significant increase in serum MDA when compared with the control group. Among various hypotheses of TBT-induced toxicity, increased oxidative stress associated with an impaired antioxidant defense status initiating a cascade of reactions has been suggested by Mitra et al. (2013), Kanimozhi et al. (2016) and Dogan et al. (2017). The decreased GPX activity shown in our study is due to excessive production of ROS by TBT leading to the exhaustion of cellular glutathione (GSH) stores and dysregulation of antioxidant enzymes as GPX, thereby leading to the impairment of antioxidant defenses. Also, the released free radicals and ROS can attack membrane phospholipids, increasing lipid peroxidation and damage to the cell membrane, this process is reflected in the elevated MDA level observed in this study.
Co-administration of green tea with TBT remarkably attenuated the effect of TBT on oxidative stress markers, indicating a profound antioxidant role of green tea as there was a highly significant increase in serum GPX and a highly significant decrease in serum MDA and TBTand green tea group when compared with tributyltin group. Green tea extract has a well-documented powerful antioxidant activity stronger for the human body than vitamin C and vitamin E as recorded by El Dine et al. (2017), Idowu (2017), Winiarska-Mieczan (2018) and Bagherpour et al. (2019). The results of the current study are supported by El Dine et al. (2017) who reported that ROS generation is a radical factor underlying TBT toxicity, and that co-administration of green tea extract ameliorated TBT induced oxidative damage.
Liu et al. (2008) reported that green tea polyphenols were effective in reducing TBT-induced oxidative damage both in vivo and in vitro. Winiarska-Mieczan (2018) reported that the antioxidant potential in blood plasma after drinking green tea increased by 34%. As antioxidants, green tea polyphenols either chelate redox-active metal ions, preventing the formation of metal-catalyzed free radicals or scavenge reactive oxygen/nitrogen species or modulate antioxidant enzymes (Idowu, 2017).
The results of the present study showed a highly significant decrease in the mean values of sperm count, viability, motility and a highly significant increase in the percent of sperm abnormal forms in the TBT treated group compared to the control group. A hummer shaped heads, banana-shaped heads, and headless tails were detected. The attributed causes for the previous changes are multifactorial. Mitra et al. (2014) reported that TBT has the potential to alter spermatogenesis by evoking either a hormonal imbalance or damage to important testicular cells such as Sertoli cells. Decreased levels of testosterone, FSH, and LH also play a role since these hormones are critical for spermatogenesis as agreed by Kanimozhi et al. (2016) who reported that the decrease in FSH might be one of the reasons causing dysfunction of Sertoli cells which play a critical role during spermatogenesis.
Upon supplementation with green tea, there was a highly significant increase in the mean values of sperm count, viability, motility, and a highly significant decrease in the percent of sperm abnormal forms as the sperms were with normal heads and tails when compared with TBT group. Abdelrazek et al. (2016), Akinwande et al. (2019) and Bagherpour et al. (2019) reported that green tea treatment significantly increased the concentration and motility of sperms and reduced the morphological abnormalities of sperms and attributed that to the prevention of excessive generation of free radicals that compromise the sperm quality by the antioxidant properties of green tea.
Light microscopic examination of H and E stained sections of the testes of the TBT treated group revealed disorganization and shrinkage of seminiferous tubules, loss of germinal epithelium, and dark pyknotic nuclei. Blood vessels in the interstitium were dilated and congested. Other sections showed marked vacuolations and acidophilic hyaline material in the interstitium in between seminiferous tubules. Similar results were reported by Mitra et al. (2013, 2014, 2017) and Kanimozhi et al. (2016).
Cytoplasmic vacuolation and degeneration could be considered an indication of germ cell necrosis according to Manivannan et al. (2009). Several germ cells had deeply stained pyknotic nuclei as a sign of germ cell degeneration as confirmed by Atli et al. (2017). The presence of wide spaces between the tubules in this study suggests the presence of interstitial edema, as reported by Kanimozhi et al. (2016). Atrophy of seminiferous tubules and the decrease in spermatogenic cells were morphological indicators of spermatogenesis failure as recorded by Uygur et al. (2014). The marked deposition of collagen fibers might be attributed to the oxidative stress induced by TBT, this explanation coincided with El-Din and Abd-El Aty (2012).
Upon administration of green tea with TBT, there was a significant improvement of all histopathological changes compared with tissues from rats treated with TBT alone. These results are supported by Heikal et al. (2014), Sheteifa and Morsy (2014), El-Beltagy et al. (2015) and Abdelrazek et al. (2016) who reported that green tea administration significantly restored the normal testicular architecture. Bagherpour et al. (2019) stated that the use of green tea improved the testicular tissue, reduced the degenerative changes in the testicles, and improved the morphometric measurements such as the height of the epithelium and the diameter of the seminiferous tubules.
Immunohistochemical staining of ki67 protein in testicular sections from TBT treated rats showed decreased expression of ki67 as a positive reaction for ki67 was only present in the nuclei of very few germinal epithelial cells compared to control group.Ki-67 is a marker of proliferation whose expression is strongly related to enhanced or suppressed proliferation and is broadly used in several studies (Takeda et al., 2011; Ukwenya, 2019). The protein expression levels of Ki-67 in the testis is one of the standard markers of spermatogenic cell proliferation according to Zhao et al. (2018).
Spermatogenic cells frequently proliferate, continuously develop, and differentiate during spermatogenesis. Several proliferation factors, including Ki-67, are involved in the proliferation and differentiation of spermatogonia in the testis as reported by Angelopoulou et al. (2008). Thus, the positive expression of Ki-67 in protein is conducive to smooth spermatogenesis. Decreased expression of ki67 in the TBT treated group indicated the decrease in proliferative activity and arrest of spermatogenesis. Previous studies mentioned the inhibitory role of TBT on cell proliferation (Mitra et al., 2014; Dogan et al., 2017; Wu et al., 2017).
Co-administration of green tea extract with TBT improved immunohistochemical results as testicular sections showed strong positive reaction seen in the nuclei of most of the germinal epithelial cells compared to TBT treated group. This improvement may be attributed to protection from oxidative damage, restoration of testicular architecture allowing for normal germ cell proliferation and normal spermatogenesis. Previous studies documented the antioxidant, anti-apoptotic effects of green tea extract on various tissues exposed to stress and damage (Heikal et al., 2014; Mosbah et al., 2015; El dine et al., 2017; Bagherpour et al., 2019). Takeda et al. (2011) reported that the intake of Theanine, one of the major amino acid components in green tea, significantly increased the number of Ki67 –labeled cells.
In the follow-up group, after the arrest of TBT treatment, there was the persistence of changes in all studied parameters. Follow up group recorded a non-significant increase in body weight, a significant decrease in absolute and relative testicular weights, a significant decrease in serum testosterone, FSH, LH, GPX, and a significant increase in serum MDA when compared with the control group. Testis still showed pyknotic dark nuclei with separation between the cells and weak positive Ki67 immunostaining. Also, there was a significant decrease in the mean values of sperm count, viability, motility, and a significant increase in sperm abnormalities when compared with the control group indicating that stopping TBT treatment alone is not enough to alleviate its toxicity or might need longer duration.
This can be explained by Si et al. (2015) who reported that TBT exposure is implicated in causing long-lasting alterations in the male reproductive system and that these changes may persist even after TBT administration ceased and far into adulthood. Moreover, Si et al. (2015) and Marques et al. (2018) reported that the tin compound is eliminated very slowly from the tissues and that TBT is a highly persistent chemical that might remain in tissues for 40–60 days after continuous exposure is ended.
Conclusions and Recommendations
Tributyltin pollution should be taken into consideration while evaluating the increasing trend of male infertility. It induces oxidative stress, cell death, alterations in the hormonal levels, leading to testicular degeneration. Green tea extract can protect against tributyltin -induced reproductive toxicity more than the arrest of exposure. It is recommended to use green tea extract for protection against tributyltin -induced reproductive toxicity. Also, further studies are needed to evaluate the effectiveness of TBTand green tea combination with longer duration, larger sample size, and larger protective doses than those used in our study. More importantly, we should increase the awareness to reduce exposure to tributyltin and pay attention to its sources to limit adverse outcomes for future generations.
Author’s Contribution
Mona El Demerdash Ibrahim and Hoda Ragab El Sayed planned this study and revised the manuscript. Marwa Gabr performed the experimental work, samples collection and all laboratory tests. Samah M. Ahmed performed the histological studies. All authors shared manuscript writing.
Conflict of interest
The authors have declared no conflict of interest.
References