Advances in Animal and Veterinary Sciences
Research Article
Molecular Detection and Immune Response of Bee Product Therapy for Induction of Endogenous Mesenchymal Stem Cells of Infertile Male Wistar Rat’s (Rattus norvegicus)
Erma Safitri*
Department of Veterinary Reproduction; Faculty of Veterinary Medicine; Universitas Airlangga, Surabaya, East Java, Indonesia, 60115.
Abstract | Molecular detection and immune response like HSP70 and PGE2 of bee product therapy has proven can for using as alternative of stem cells therapy of infertile male rat (Rattus norvegicus). The stem cells therapy can cope with various diseases including infertility caused by the degenerative conditions of the testis. Nevertheless, the complexity of the isolation method, in vitro culture process and transplant procedure with booster treatment are very expensive. The innovation was needed as an effort to alternative through of induction of endogenous stem cells. Forty rats were devided 4 groups: Normal rats, without honey (T-); infertile rats, without honey (T+); infertile rats were given 30% honey (v/v) for 10 days (T1) and infertile rats were given 50% honey (v/v) for 10 days (T2). The result, HSP70 and PGE2 expression as a immune response for regenerative signal in group, T-, T+, T1 and T2 respectively is 0.15a ± 0.5; 3.15b ± 0.4; 2.95b ± 0.35 and 0.75a ± 0.15 (for HSP70) and 1.15b ± 0.00; 0.19a ± 0.53; 0.27a ± 0.39 and 2.95c ± 0.45 (for PGE2). Number of Leydig and spermatogenic cells (spermatogonia, spermatocyte primer-seconder and spermatid) in T+ was significantly decreased (p<0.05) when compared with T-, T1 and T2, while T2 didn’t show decreased significantly (p>0.05) compared with T-. It could be concluded, that Molecular detection and immune response of bee product therapy was based on high increase of PGE2 and few increase of HSP70 in testicular tissue and then can rescue of infertile male rat with degenerative testis.
Keywords | HSP70, PGE2, Honey, Endogenous stem cells, Infertile male rat
Received | June 20, 2020; Accepted | September 3, 2020; Published | November 15, 2020
*Correspondence | Erma Safitri, Department of Veterinary Reproduction; Faculty of Veterinary Medicine; Universitas Airlangga, Surabaya, East Java, Indonesia, 60115; Email: erma-s@fkh.unair.ac.id
Citation | Safitri E (2020). Molecular detection and immune response of bee product therapy for induction of endogenous mesenchymal stem cells of infertile male wistar rat’s (Rattus norvegicus). Adv. Anim. Vet. Sci. 8(12): 1388-1393.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1388.1393
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Safitri. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Testis is the most important organ of male reproduction for producing of sperm and testosterone as main hormone reproduction and libido expression. One of the major cases of infertility in male can be caused by the degeneration of the testis tissue, specifically due to malnutrition (Safitri et al, 2016a; Prasetyo and Safitri, 2016). Therapy was using stem cells in now and next few decades is very interesting and greatly increased sharply, because stem cells potential is very promising for utilization as a treatment (Lee et al., 2016). The stem cells therapy can cope with various diseases (Bali et al., 2017) including infertility caused by the degenerative conditions of the testis (Safitri et al., 2016b).
Nevertheless, the complexity of the isolation method, in vitro culture process and transplant procedure with booster treatment are very expensive (Safitri et al., 2019). The innovation was needed as an effort to alternative through of induction of endogenous stem cells. Therapeutic innovation was done using stem cell induction and this resulted in auto-regeneration in the testis of the mice (Mus musculus), especially the seminiferous tubular cells that reproduce spermatozoa by using bee products such as honey (Safitri et al., 2016b). The honey is a nutrient gotten from bees (Hasib et al., 2017). It could serve as an antioxidant and as an antibacterial (Denga et al., 2018). Antioxidant is an important substance that protects individuals from free radicals such as reactive oxygen species (ROS). Adequate consumption of an antioxidant can reduce the prevalence of cardiovascular failure, cancers, digestive tract disorder, cataract and other degenerative diseases (Hasib et al., 2017; Prasetyo and Hestianah, 2017), it also leads to degenerative testicular. Consumption of honey products is also effective in the treatment of diarrhea with low immune response (Prasetyo and Safitri, 2016) and the reproductive system disorder as well (Safitri et al., 2016a, b).
According to other researchers, the administration of honey products can lead to auto-mobilization, increased immune response (Hasib et al., 2017). It can also lead to the differentiation of the follicle and the ephitelial of the intestines of the female mice (Mus musculus) and the degeneration of the ovaries and intestines (Prasetyo and Safitri, 2016). The increased immune response (Prasetyo and Hestianah, 2017), the presence of automobilization and the differentiation of the stem cells is takes place in the body leading to the regeneration of the lamina propria, epithelial villi of the intestines, follicle and corpus luteum of the ovary (Prasetyo and Safitri, 2016). The presence of auto-mobilization and the differentiation of the stem cells that takes place in the body also leads to auto-regeneration and this takes place in the seminiferous tubules found in the testicles of the mice as a result of malnutrition caused by the use of honey product (Safitri et al., 2016b).
As a conceptual solution, this study aimed to explore the benefits of the honey as bee product from the use of forest bee (Apis dorsata), to induce of stem cells endogen of male rat as an alternative for stem cells therapy (without exogen injected). The induce of stem cells endogen can regenerative of testicles and sertoli cells and then provides support, nutrients and other environmental factors for the young spermatozoa and this permits spermatogenesis and conception to take place.
The carried out an investigation to prove that the bee product from Apis dorsata was effective for the treatment used to induce of stem cells endogen and the regeneration of the seminiferous tubules and leydig cells of male rats (Rattus norvegicus).
MATERIALS AND METHODS
Ethical approval
The present study was approved by the ethical committee through the Ethical Clearance institution (Komisi Etik Penelitian), Animal Care And Use Committee (ACUC), Faculty Of Veterinary Medicine, , University of Airlangga, Surabaya, Indonesia (Number 239-KE 2018).
Degenerative treatment of testicle tissue
Degeneration of testicles was done by carrying out a research work using a male rat model. Some of the male rats went without food for five days but were given water throughout those five days (Safitri et al., 2016b). The rats used in this case study were very healthy, 8-10 week old male rats (Rattus norvegicus), wistar strain with a body weight of 250-300g each. The rats were placed in individual plastic cages in the Experimental Animal Laboratory at the Faculty of Veterinary Medicine, University of Airlangga. They rats were divided into 4 groups, each group consisting of 10 rats. Group 1 was named the negative control group (T-); they were properly fed with not given bee product. Group 2 was named the positive control group (T+); they weren’t given food and bee product. Group 3 was named the trial group 1 (T1); they weren’t given any food but were fed with 30% (v/v) of bee product for 10 days. Group 4 was named the trial group 2 (T2); they went without food but were given 50% (v/v) of bee product for 10 days.
Identifying the testicle tissue regeneration
Identifying the testicle tissue regeneration was done by histopathological examination. The histopathological preparation was done using rats testicular fixation that was submerged in 10% formalin. Subsequent rat testicles dehydrated in alcohol solutions with higher concentrations from 70%, 80%, 90% and 96%. After which the testicles were submerged in xylol solution. Embedding was performed using liquid paraffin and the rat testicles were put into molds containing liquid paraffin. An incision was made using a microtome and mounted glass objects after sectioning was performed and stained. On the contrary, the removal of paraffin from the testicles was performed after staining was done by xylol, and then put into a solution of alcohol. This decreases the concentration from 96% to 90% to 80% and 70%, after which it was submerged in hemaxtocylin eosin (HE) staining procedure.
Finally, after staining is done, the testicles were submerged into alcohol with an increasing concentration from 70% to 80%, to 90% and 96% and then put into xylol solution. The preparation was then covered with a glass cover and mounted with Canada balsam. Histopathological examination of seminiferous tubules tissue, leydig cells, spermatogonia, primary and secondary spermatocyte and spermatid cells regenerate in the testicle tissue was also carried out. Regeneration is based on the existing histological description which is done by using a light microscope with a magnification of 200 (Safitri et al., 2016a).
Immunohistochemical (IHC) of HSP70 and PGE2
The IHC observation was performed to determine the expression of Heat shock protein 70 (HSP70) and Prostaglandin E2 (PGE2). Previously, before to IHC were made histological preparation, by way of an incision is made transversely testicular tissue from paraffin blocks. Further examination by making outward through IHC techniques using HSP70 monoclonal antibody (Cat #MA3-006 50µl, Thermo Fisher Scientific, Pittsburgh, PA, USA) and PGE2 (PTGER3 Polyclonal Antibody PGE2 for IHC 50µl, Thermo Fisher Scientific, Pittsburgh, PA, USA).
This is done to determine the expression of HSP70 and PGE2 were made a regular light microscope with a magnification of 200 times and the expression of each variable is indicated by the number of cells with brownish discoloration chromogen in each incision (Kumar and Rudbeck, 2009). The observation used regular luminescence microscope Nikon H600L which is equipped with digital camera DS Fi2 300 megapixels and image processing software and cell count Nikon Image System.
Statistical analysis
The cell numbers of the leydig, spermatogonia, spermatocyte primary-secondary and spermatid cells and the score of HSP70 and PGE2 in the testicle tissue of the male rats were statistically analyzed using SPSS 17 for windows XP. The confidence level was 99% (α = 0.01) and the level of significance was 0.05 (p=0.05). The steps involved in the hypothesis are as follows; the normality data test, the kolmogorof smirnov test, the homogeneity variance test, analysis of the variants and the post hoc test using the Turkey HSD 5% as the least significant difference test.
RESULTS AND DISCUSSION
The results detail of the study was as follows: the effectively of bee product was based on: HSP70 and PGE2 expressions and regeneration of tisticular tissue with improvement of number of Leydig and spermatogenic cells (spermatogonia, spermatocyte primer-secondary and spermatid).
The regeneration was observed through of histopathology anatomy (HPA) method with hematoxylin eosin (HE) staining. The microscopic observation was showed that there an improvement in the seminiferous tubules with 50% (v/v) bee product treatment (T2). This improvement was based on the regeneration from the seminiferous tubules, leydig, sertoli, spermatogonia, primary-secondary spermatocyte, and spermatid cells (Figure 1D, Table 1). Furthermore, the improvement can be compared with the control negative group (T-) that did not experience degeneration, this indicates normal condition (Figure 1A). On the testis degeneration with 30% (v/v) bee product treatment (T1), did not show any improvement from testicle tissue (Figure 1C). The absence of seminiferous tubules improvement, is an indication of seminiferous tubules damage. The damage description can be compared with the control positive group (T+), the male rat with testicular degenerative (Figure 1B).
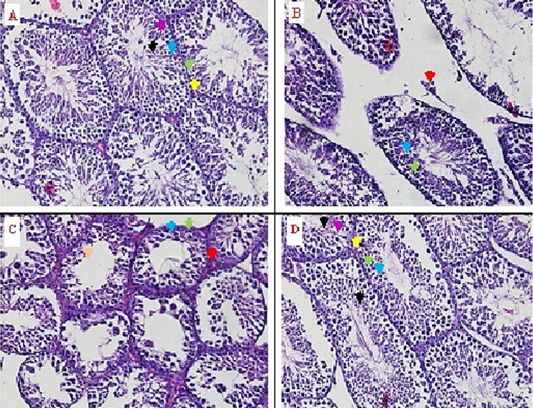
Figure 1: The identification of seminiferous tubule tissue and leydig cells from rat testes through histopathology anatomy (HPA) method with hematoxylin eosin (HE) staining on some treatments. Yellow arrow () = normal leydig cells, red arrow () = nekrosis leydig cells, green arrow () = spermatogonia cells, blue arrow () = spermatocyte primary-secondary, black arrow () = normal spermatid cells, orange arrow () = nekrosis spermatid cells, purple arrow () = sertoli cells.
In the control positive group (T+) there was a significant decrease in the number of the four cells (Leydig, spermatogonia, primary-secondary spermatocytes and spermatid cells) (p<0,05) was compared with the three other groups (T-, T1 and T2) (Table 1, Figure 1). Table 1 was showed that 30% (v/v) the bee product treatment (T1) still show significant difference (p<0,05) was compared with the control negative group and the treatment was 50% (v/v) bee product treatment (T- and T2).
Based on Table 1, the treatment of 50% (v/v) bee product (T2) was decreased not significantly different (p>0,05) compared with the negative control group (T-) based on the number of the spermatogonia cells, the primary-secondary and spermatid. However, the treatment of the 50% (v/v) bee product (T2) still was showed significant decrease (p <0.05) compared with negative control group (T-) both in the number leydig cells and the spermatid cells.
Furthermore, the increased of immune response for regenerative signal based on Hsp70 expression, in the normal control group (T−) was on the score 0.15a ± 0.5. The positive control group (T+) was on the score 3.15c ± 0.4. The group 30% (v/v) bee product (T1) was on the score 2.95c ± 0.35. The group use 50% (v/v) bee product (T2) was on the score 1.75b ± 0.15 (Table 2).
Table 1: Number of leydig cells, spermatogonia, spermatocyte primary-secondary and spermatid cells in some treatments.
| No | Treatments | Average leydig cells±SD | Average spermatogonia± SD | Average spermatocyte Pri-Sec ±SD | Average spermatid ±SD |
| 1. | Negative control group (T-) : Normal rat, without honey |
15.75 d ± 0.50 |
34.25d ± 2.45 |
65.25d ± 1.60 |
75.50d ± 1.40 |
| 2. | The positive control group (T+) : infertile rats, without honey |
4.25a ± 1,85 |
13.55a ± 1,57 |
30.44a ± 1.73 |
13.15a ± 1.70 |
| 3. | Trial group 1 (T1) : infertile rats were given 30% honey (v/v) for 10 days |
6.85b ± 1.60 |
23.53b ± 1.34 |
42.35b ± 1.40 |
26.53b ± 1.34 |
| 4. | Trial group 2 (T2) : infertile rats were given 50% honey (v/v) for 10 days. |
10.75 c ± 0.50 |
39.75cd ± 1.35 |
58.75cd ± 1.54 |
49.65c ± 1.75 |
a,b,c,d Different superscripts in the same columnwas significantly different (P<0,005).
Table 2: Score of HSP70 and PGE2 in some treatments.
| No | Treatments | Average Hsp70 expression score ± sd | Average PGE2 expression score ± sd |
| 1. | Negative control group (T-) : Normal rat, without honey |
0.15a ± 0.5 |
1.15b ± 0.00 |
| 2. | The positive control group (T+) : infertile rats, without honey |
3.15c ± 0.4 |
0.19a ± 0.53 |
| 3. | Trial group 1 (T1) : infertile rats were given 30% honey (v/v) for 10 days |
2.95c ± 0.35 |
0.27a ± 0.39 |
| 4. | Trial group 2 (T2) : infertile rats were given 50% honey (v/v) for 10 days. |
1.75b ± 0.15 |
2.95c ± 0.45 |
a,b,c,d Different superscripts in the same columnwas significantly different (P<0,005).
Other than that, the increased of immune response, also based on PGE2 expression, in the normal control group (T−) was on the score 1.15b ± 0.00. The positive group (T+) was on the score 0.19a ± 0.53. The group 30% (v/v) bee product (T1) was on the score 0.27a ± 0.39. The group use 50% (v/v) bee product (T2) was on the score 2.95c ± 0.45 (Table 2)
The results of this study showed that the administration of a dose of 50% (v/v) bee product from Apis dorsata in drinking water for 10 days in the T2 group can be used to treat male rats with seminiferous tubules and leydig cells degeneration. The effectiveness of these bee product form Apis dorsata in this study is based on several things such as: The identification of the seminiferous tubules and the leydig cells regeneration. These include the number of leydig cells, the spermatogonia cells, the primary-secondary spermatocyte, the spermatid cells, and increased of immune response such as HSP70 and PGE2 for induced of endogenous stem cells.
The regeneration from the seminiferous tubules and the leydig cells were marked with tissue that was intact. In this result, regeneration was observed through histopathology anatomy (HPA) method with hematoxylin eosin (HE) staining. The microscopic observation showed that there was an improvement in the seminiferous tubules with 50% (v/v) bee product (T2). This improvement was based on the regeneration from the seminiferous tubules, the leydig, the sertoli, the spermatogonia, the primary-secondary spermatocyte, and the spermatid cells (Figure 1D, Table 1). Furthermore, the improvement can be compared with the control negative group (T-) that did not experience degeneration, this indicates normal conditions (Figure 1A). On the testis degeneration with the treatment of 30% (v/v) bee product (T1), did not show any improvement from testis tissue (Figure 1C). The absence of improved seminiferous tubules, is an indication of damaged seminiferous tubules. The damage description can be compared with the control positive group (T+), the male rat with testicular degenerative (Figure 1B).
The damage also occurred in the leydig cells, the sertoli cells and the spermatid cells, besides that there was a decrease in the number of spermatogonia cells, primary and secondary spermatocyte cells (Figure 1B, Table 1). In the 30% (v/v) bee product (T1), the seminiferous tubules were still damaged, the leydig cells, the sertoli cells and the spermatid cells were still damaged and there was a decrease in the number of cells, as well as the spermatogonia cells, the primary-secundary spermatocyte cells as shown in (Figure 1C, Table 1).
Regeneration of testis tissue was observed based on the number of leydig cells, the spermatogonia, the primary-secondary spermatocyte, and the spermatid cells. From the Table 1, it could be concluded that the treatment of 50% (v/v) bee product (T2) not significantly lower (p>0,05) compared to the control negative group (T-) based on the number of the spermatogonia, primary and secondary cells. But, the treatment of 50% (v/v) bee product (T2) still was showed a decrease and significant different (p <0.05) than the negative control (T-) on the number of leydig and spermatid cells. This suggests that 50% (v/v) bee product (T2) has already shown results from the seminiferous tubular tissue, but the number of Leydig cells are not yet equal to the normal group (T-). This will affect the levels of testosterone produced, given that the Leydig cells are cells that play a role in producing the male hormone testosterone (Hafez, 2013). Similarly, the number of spermatid cells has not been the same as with the normal group (T-). This will affect the number of spermatozoa cells formed from morphological changes of spermatid cells into spermatozoa cells.
Furthermore, increased of immune response such as HSP70 and PGE2 for inducing of endogenous stem cells can occur due to mobilized of stem cells toward the defect. The process of mobilization can occur in several ways, one of which is an increase due to the immune response was induced inflammatory reaction due to injury signals such as NFkB, Wnt through beta-catenin and Cytokines of tissue damage (Eleotério et al., 2016). In this study, injury due to malnutrition signal causes an increase in Hsp70 and decreases PGE so that damage to the testicular tissue can not be inevitable. The damage in the testicular tissue is the cause of the disruption of the absorption of food that is needed by testicular tissues including the tubulus seminiferus as the primary source of the spermatogenesis process (Tripathi et al., 2015).
This condition was need of rescue, especially improvements in testicular tissue as the primary tissue of male reproductive organ. PGE2 have long been known to have cytoprotective effects on the testicular epithelium. Their cytoprotective effect appears to result from a complex ability to stimulate mucosal mucus to increase mucosal blood flow, to limit back diffusion of acid into the epithelium (Barker, 2014). Furthermore, the stem cells were proliferated continually to supply cells that then differentiate into leydig, sertoli and spermatocyte cells. This is in accordance with the opinion, that bee product from Apis dorsata will cause the stem cells develop rapidly differentiate into cells that are needed as a response of the defect and enhancement of the immune response (Dussaubat et al., 2012). Regeneration of testicular tissue, such as: Intact of testicular tissue and can be observed through the method of histopathology anatomy (HPA) with HE staining. Microscopic examination showed that the group of 50% (v/v) bee product (T2), leading to the occurrence of testicuar tissue repair. Improvements are identified based on the regeneration of testicular tissue with spermatogenesis process can occure. Overview of these improvements can be compared with a control negative group (T−) who did not experience testicular degeneration, which remains in normal condition with intact of testicular tissue. As for the group of 30% (v/v) bee product (T1) does not indicate the occurrence of testicular tissue repair. Not the improvement in the form of testicular that are no longer intact (broken). Figure of the damage can be compared with the positive control group (T+) with testicular degeneration. The positive control group (T+), testicular was congested and hemorrhagic extensive, also visible hemosiderin (yellow brown) due to blood cell lysis with fibrin deposition (baby color pink) indicating that chronic congestion has occurred. The group use 30% (v/v) of bee product (T1), the testicular does not regenerate, it appears there is congestion along hemosiderin expression and are still widely hemorrhagic. Treatment of 50% (v/v) concentration bee product for 10 days in female rat with degenerated testicular can show (a) mobilization of endogenous stem cells (HSCs), (b) increased immune response in the form of few increase in the expression of Hsp70 and high increased of PGE2 by immunohistochemistry in testicular tissue; (c) regeneration of testicular tissue, such as: intact of testicular tissue, although there is still little hemorrhagic and congestion but hemosiderin expression and fibrin deposition has not looked back.
Furthermore, the positive control group (T+) experienced a decreased libido (based on decrease of Leydiq cells), because the adrenal cortex became ineffective in producing dehydroepiandrosterone (DHEA) due to malnutrition. Low levels of DHEA in the blood can be cause of decreased body stamina, fatigue, and also decreased libido. DHEA was produced by the renal adrenal cortex (Heckbert and Heian, 2002) and the Leydig cells (Hafez, 2013) is the most potent precursor of steroid hormones such as testosterone. It also occurs in the degenerative group of testes who have received 30% (v/v) bee product (T1) for 10 days. This suggests that 30% dose of honeybee has not been able to restore libido as in the negative control group (T-).
DHEA was increased as a precursor of steroid hormones such as testosterone, it is also responsible for acts as an enzyme inhibitor of fat metabolism through of glucose 6-phosphat dehydrogenase inhibision, which has a role as a biocatalyst that changes glucose to fat. The increase in DHEA allows for an increase in the amount of free ATP in the body, and then increasing the body stamina (Joshi et al., 2018; Maggio et al., 2014). libido and fertility (Hafez, 2013).
CONCLUSIONS AND RECOMMENDATIONS
In conclusion, the present results indicates that the effectiveness of the bee product from Apis dorsata usage therapy is based on: molecular detection and immune response of bee product therapy was based on high increase of PGE2 and few increase of HSP70 expression in testicular tissue and then can rescue for the regeneration of seminiferous tubules and leydig cells, improvement of the Leydig cell number, spermatogonia cells, primary-secondary spermatocytes and spermatid cells.
ACKNOWLEDGMENTS
The study was supported by funding from the Directorate General of Higher Education (DIKTI) 2018. The National Education Ministry. Republic of Indonesia. Grant number: 005/ADD/SP2H/LT/DRPM/VIII/2018.
AUTHOR’S CONTRIBUTIONS
ES conceptualization, methodology, data analysis, research and ethical clearance preparation of the research equipment, observation of Histopathology Anatomy (HPA) and Immunohistochemical method (HSP70 and PGE2), draft for manuscript preparation (wrote of the paper). Preparation of animal experimental, and corresponding author.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES





