Advances in Animal and Veterinary Sciences
Research Article
Effect of Mycotoxins on the Spermatozoa and Embryos of Animals
A.V. Tkachev1*, O.L. Tkacheva1, T.V. Zubova2, V.A. Pleshkov2, O.V. Smolovskaya2
1FSBEI HE Belgorod State Agricultural University, Belgorod, Russia; 2FSBEI HE Kuzbass State Agricultural Academy, Kemerovo, Russia.
Abstract | Of the aim of the current study is to study the cytotoxic effect of Zearalenone and T-2 toxin on the sperm of bulls and stallions following the Kharkov technology of sperm obtaining and cryopreservation. Invitro toxicity effect of various concentrations of Zearalenone and T-2 toxin (0.5 mM to 0.01 mM) on the membrane integrity, as well as on the biological indicators of the spermatozoa of bulls and has been shown. Our results revealed that after adding 0.5 mM and 0.25 mM of Zearalenone, the biological activity of native sperm cells after one hour did not change, and after thawing, it decreased by 19.4 % (P < 0.01) and 12.2 % (P < 0.05); respectively, compared to non-treated negative control. On the other hand, the T-2 toxin at the concentration of 0.5 mM and 0.25 mM reduced the biological activity of native sperm cells after one hour of incubation by 10.14 % (P < 0.01) and 8.03 % (P < 0.05), respectively and their activity after thawing decreased by 60.28 % (P < 0.001) and 52.7 % (P < 0.001); respectively, compared to non-treated negative control. In this study, the lowest concentration that gave a veracious effect on the characteristics of the sperm was 0.01 mM of Zearalenone and the T-2 toxin, and after thawing only a slight decrease in the activity and survival rate of sperm that was 2.3 and 6.5 %, respectively. While at the dosage of 0.01 mM of Zearalenone and T-2 toxin, a veraciously significant decrease in biological survival rate and the absolute survival indicator of native sperm by 8.7 and 4.3 % (P < 0.05), respectively. The cytotoxic effect of Zearalenone and T-2 toxin on the sperm of bulls and stallions in vitro after cryopreservation has been studied in vitro after cryopreservation. It has been found that the minimum cytotoxic dosage of Zearalenone and T-2 toxin during the cryopreservation was 0.01 mM. Likewise, the species differences have been shown in the sensitivity of the spermatozoa of bulls and stallions for the cytotoxic effect of Zearalenone and T-2 toxin during cryopreservation, which is evidence to the fact that complex addition of Zearalenone and T-2 toxin to the diluent has lower toxicity to the plasmalemma, acrosome, and the biological parameters of the spermatozoa of bulls than adding T-2 toxin alone, which is not observed in the sperm of stallions.
Keywords | Bulls, Cytotoxic effect, Mycotoxins, Sperm cells, Stallions, T-2 toxin, Zearalenone
Received | August 01, 2020; Accepted | August 7, 2020; Published | August 28, 2020
*Correspondence | A.V. Tkachev, FSBEI HE Belgorod State Agricultural University, Belgorod, Russia; Email: elen.ulrich@mail.ru
Citation | Tkachev AV, Tkacheva OL, Zubova TV, Pleshkov VA, Smolovskaya OV (2020). Effect of mycotoxins on the spermatozoa and embryos of animals. Adv. Anim. Vet. Sci. 8(s3): 47-55.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s3.47.55
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Tkachev et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Recently, several reports have been focused on the decline of productivity either in humans or animals (Luconi et al., 1999, 2004; Tesarik et al., 2000; Tsakmakidis et al., 2006). In different countries, every third to fifth couple is forced to resort to the services of theriogenology clinics to give birth for a child (Arvidson et al., 1989). With that, the theriogenology clinics face the same problems as the livestock breeding including reduced efficiency of sperm cryopreservation (Milano et al., 1991; Ronquist and Brody, 1985; Savchenkova and Vasilieva, 2016; Sousa et al., 2002). The reduced efficiency of sperm cryopreservation in humans and animals may be caused by the presence of even the permissible level of mycotoxins in food products for humans and animal (Diaz, 2006; Tkachev, 2015a).
One of the factors that may reduce the biological indicators of spermatozoa may be the effect of permissible concentrations of mycotoxins in the feed in case of prolonged chronic toxicosis passing without obvious clinical symptoms (Diaz, 2006; Tkachev, 2014a). A group of researchers in 1994 proved that prolonged animal intake of feed with acceptable concentrations of Zearalenone into provoked its accumulation in the tissues of the prostate, testes, ovaries in females, kidneys, hypothalamus and urinary excretion in the amount of up to 7 mM (Erasmuson et al., 1994). Hence, the probability of high concentration of mycotoxins getting into the sperm during ejaculation is high, which can reduce the biotechnological fitness of sperm cells to cryopreservation (Tkachev, 2013a). In 2011, Italian researchers proved the negative effect of Zearalenone and its derivatives on the acrosome reaction and the mobility rates of freshly obtained stallion sperm in vitro (Filannino et al., 2011). Earlier, the negative effect of mycotoxins in vitro had been shown on sperm cells of rams and boars (Farnworth and Trenholm, 1983). Previous studies reported that massive abortion in mares in the USA between 40 and 100 days of pregnancy, caused by the intake of allowed dosages of Zearalenone within the feed within two weeks; which amounted to 336 million U.S. dollars (Diaz, 2006). Collectively, these studies highlight the significance effect of mycotoxins on the biological indicators of spermatozoa after cryopreservation.
Likewise, the high contamination of wheat, barley, maize, etc. with mycotoxins (Tkachev, 2014b), has negative effect on the hormonal and cytogenetic status of animals and humans (Carlini et al., 1997; Francavilla et al., 2003; Lukoseviciute et al., 2007; Tkachev, 2013b; Tkachev, 2015b), along with low cryoresistance of spermatozoa (Graham, 1996). So, there is an urgent need to determine the minimum concentration of mycotoxins that affects the biological properties of germ cells in stallions.
Animal breeding worldwide including Russia, a sharp decrease in the reproductive qualities for males and females of farm animals is observed. Previous studies showed that average across the horse breeding industry, the number of actually born foals per 100 mares did not exceed 50 which means that today only three out of 12 officially registered breeds have sufficient breeding reproductive population (Tkachev, 2013b; Tkachev, 2014b). Therefore, the primary scientific and practical task of the biology of reproduction is to increase the yield of young animals. It is known that up to half of the stud males both in Russia and worldwide provide biotechnologic sperm that either dies in the process of freezing and thawing or loses its fertilizing ability after depreservation (Casey et al., 1997; Katila, 2001; Magistrini et al., 1996; Mottershead, 2000; Tkachev, 2014b).
The current study aims to determine the invitro cytotoxic effect of various dosages of Zearalenone and T-2 toxin, which would reduce cryoresistance of the spermatozoa of bulls and horses following the Kharkov technology of sperm cryopreservation.
Materials and Methods
The current study was performed in Russia (the Belgorod and Kemerovo regions) on separated ejaculates of six stud stallions; three from the Belgian breed and three from the Ukrainian riding breed, and three stud bulls of the black motley breed. The sperm of the stud bulls and stallions was obtained and cryopreserved following the Kharkov technology developed by the authors (Tkachev, 2013a).
The fundamental difference of the Kharkov technology for sperm cryopreservation is the use of an experimental device for two-step freezing with the possibility of fieldwork, and a large amount of semen dose 4 to 5 ml (instead of 0.25 – 0.5 ml used in the Western European technologies). The ejaculates were obtained twice a week. After obtaining and before cryopreservation, Zearalenone (Sigma, USA) and T-2 toxin (Sigma, USA) were introduced into the sperm diluent at a concentaration 0.5– 0.01 mM. Zearalenone (C18N22O5, molecular weight 318.36) is a nonsteroidal mycotoxin, which is produced by the fungi of the genus Fusarium; and has an estrogenic action and causes abortion, infertility, gynecomastia, and appearance of mammary gland tumors either in human or animals (Filannino et al., 2011). The T-2 toxin (C24N34O8, molecular weight 466.52) is a highly toxic trichothecene produced by the fungi of genus Fusarium; when injected into the human body, it provokes the development of alimentary toxic aleukia and polyorganic pathology; in some historical events. The diluent for sperm was the SMED formulation (100 ml of distilled water, 37 mM of NaCl, 10 mM of KCl, 0.07 mM of KH2PO4, 35.7 mM of NaHCO3, 2.4 mM of MgSO4, 10 mM of HEPES, 1.7 mM of CaCl2, 84.3 mM of fructose, 5.5 mM of glucose, and 0.3 g of bovine serum albumin, pH 7.2), and upon freezing, the penetrating cryoprotectant glycerol (Sigma, USA) in the amount up to 7 % of the final volume was added to the diluent (Backman et al., 2004). To establish the cytotoxic effect of Zearalenone and T-2 toxin, each ejaculate was divided into four equal parts where the first part was used as non treated negative control, the second one Zearalenone was added, the third one T-2 toxin was added, and the fourth one Zearalenone and T-2 toxin were added simultaneously in equal amounts. After that, the results obtained and compared on native freshly diluted and thawed sperm. In the native (freshly obtained) and thawed sperms using conventional methods (GOST 20909.4-75; GOST 24168-80; GOST 26030-83; Filannino et al., 2011; Tkachev, 2013a), the main indicators generally adopted in biology of animal reproduction were determined. The activity of the sperm cells was determined visually under Jenaval light microscope (Germany) in points (1 point was equal to 10 % of the live sperm cells with the uniform rectilinear translation) under the magnification of 10 – 20×. The survival rate of the sperm cells was determined in hours in a thermostat at 37 °C which considers the most important biological indicator used for predicting the result of insemination. A 5% decrease in the sperm cells activity in a thermostat was the final point of determining the survival rate in hours. The absolute index of survival rate is calculated, which objectively characterizes the preservation of sperm cells activity during exposure to the conditions in the thermostat; and it is expressed in arbitrary units and calculated according to the standard formula of the state standards (GOST 20909.4-75; GOST 24168-80; GOST 26030-83; Tkachev, 2013a).
The damage of the sperm cells’ membranes under the action of mycotoxins after cryopreservation was examined using SU8000 Hitachi electronic microscope (Japan) at the magnification of 3,000 – 3,500 times.
The statistical processing was performed using generally accepted methods of variation statistics, the statistical significance of differences was evaluated according to Student t-criterion (Plokhinsky, 1969). The tables show mean (M) and mean deviations (±m). The dispersion analysis was performed using a specialized application program package SPSS for Windows (IBM, USA).
RESULTS
To determine the minimal dosages of mycotoxins that can reduce the basic biological parameters of sperm cells in stallions after cryopreservation, the in vitro effect of Zearalenone and T-2 toxin was studied for determining the quantitative and qualitative indicators for native and thawed sperm cells on diluted ejaculates (Table 1).
Table 1: The cytotoxic effect of Zearalenone and T-2 toxin on the performance of native and thawed spermatozoa (M ± m, p= 11).
| Indicator | Toxin | The amount of toxin | ||||
| 0.5 mM | 0.25 mM | 0.1 mM | 0.05 mM | 0.01 mM | ||
| Biological indicators of the native sperm cells in horses | ||||||
| Sperm cells activity (after one-hour exposure), points | without toxins | 5.82 ± 0.12 | 5.73 ± 0.14 | 5.91 ± 0.09 | 5.82 ± 0.12 | 5.91 ± 0.09 |
| Zearalenone | 5.82 ± 0.12 | 5.73 ± 0.14 | 5.91 ± 0.09 | 5.82 ± 0.12 | 5.91 ± 0.09 | |
| T-2 toxin |
5.23 ± 0.10b |
5.27 ± 0.12a |
5.45 ± 0.16a |
5.41 ± 0.09a |
5.64 ± 0.15 | |
| Zearalenone + T-2 |
4.91 ± 0.09c |
5.09 ± 0.16b |
5.18 ± 0.18b |
5.27 ± 0.10b |
5.55 ± 0.16 | |
| Sperm cells survival rate, hours | without toxins | 5.09 ± 0.09 | 5.18 ± 0.12 | 5.09 ± 0.09 | 5.18 ± 0.12 | 5.18 ± 0.12 |
| Zearalenone |
4.55 ± 0.21a |
4.64 ± 0.20a |
4.91 ± 0.09 | 5.18 ± 0.12 | 5.18 ± 0.12 | |
| T-2 toxin |
3.91 ± 0.16c |
4.00 ± 0.13c |
4.45 ± 0.16 b |
4.64 ± 0.15a |
4.91 ± 0.09 | |
| Zearalenone + T-2 |
3.09 ± 0.16 c |
3.18 ± 0.18 c |
3.55 ± 0.16 c |
3.91 ± 0.09 c |
4.73 ± 0.14 a |
|
| The absolute survival rate, conventional units | without toxins | 14.77 ± 0.08 | 14.86 ± 0.07 | 14.82 ± 0.10 | 14.91 ± 0.09 | 14.91 ± 0.09 |
| Zearalenone |
14.32 ± 0.12 b |
14.41 ± 0.13 b |
14.55 ± 0.13 | 14.64 ± 0.15 | 14.91 ± 0.09 | |
| T-2 toxin |
10.41 ± 0.06 c |
10.55 ± 0.11 c |
11.00 ± 0.24 c |
11.91 ± 0.21 c |
14.45 ± 0.16 a |
|
| Zearalenone + T-2 |
8.68 ± 0.08 c |
8.77 ± 0.08 c |
9.14 ± 0.14 c |
10.05 ± 0.13 c |
14.27 ± 0.24 a |
|
| Biological indicators of thawed sperm cells in horses | ||||||
| Sperm cells activity (after one-hour exposure), points | without toxins | 3.55 ± 0.16 | 3.36 ± 0.15 | 3.82 ± 0,12 | 3.91 ± 0.09 | 3.91 ± 0.09 |
| Zearalenone |
2.86 ± 0.10b |
2.95 ± 0.05 a |
3.27 ± 0.14b |
3.45 ± 0.16 a |
3.82 ± 0.12 | |
| T-2 toxin |
1.41 ± 0.06c |
1.59 ± 0.11c |
1.73 ± 0.12c |
2,27 ± 0.14c |
3.45 ± 0.16 | |
| Zearalenone + T-2 |
0.59 ± 0.06c |
0.77 ± 0.08c |
0.95 ± 0.08c |
1.50 ± 0.12c |
3.36 ± 0.15a |
|
| Sperm cells survival rate, hours | without toxins | 3.91 ± 0.16 | 4.09 ± 0.09 | 4.18 ± 0.12 | 4.09 ± 0.09 | 4.18 ± 0.12 |
| Zearalenone |
2.82 ± 0.18c |
3.00 ± 0.13c |
3.55 ± 0.16b |
3.64 ± 0.15a |
3.91 ± 0.09 | |
| T-2 toxin |
1.86 ± 0.07c |
1.95 ± 0.05c |
2.41 ± 0.15c |
2.73 ± 0.14c |
3.64 ± 0.15a |
|
| Zearalenone + T-2 |
1.09 ± 0.09 c |
1.18 ± 0.12 c |
1.41 ± 0.11 c |
1.73 ± 0.12 c |
3.55 ± 0.16 b |
|
| The absolute survival rate, arbitrary units | without toxins | 12.05 ± 0.24 | 12.18 ± 0.27 | 13.05 ± 0.11 | 13.32 ± 0.14 | 13.21 ± 0.17 |
| Zearalenone |
6.45 ± 0.08 c |
6.59 ± 0.11 c |
7.41 ± 0.25 c |
10.05 ± 0.33c |
13.14 ± 0.21 | |
| T-2 toxin |
3.59 ± 0.09 c |
3.73 ± 0.10 c |
4.23 ± 0.21 c |
5.32 ± 0.18 c |
10.32 ± 0.1c |
|
| Zearalenone + T-2 |
1.86 ± 0.07 c |
2.09 ± 0.15 c |
2.77 ± 0.16 c |
3.45 ± 0.16 c |
10.09 ± 0.06 c |
|
Note: a, P< 0.05; b , P < 0.01; c , P < 0.001 (compared to the reference without toxins).
Table 2: The cytotoxic effect of Zearalenone and the T-2 toxin on the performance of native and thawed spermatozoa of bulls (M ± m, p= 11).
| Indicator | Toxin | The amount of toxin | ||||
| 0.5 mM | 0.25 mM | 0.1 mM | 0.05 mM | 0.01 mM | ||
| Indicators of the native sperm of bulls | ||||||
|
Sperm cells activity (after one-hour exposure), points |
without toxins | 6.2 ± 0.13 | 7.8 ± 0.13 | 7.3 ± 0.15 | 7.9 ± 0.1 | 8.1 ± 0.18 |
| Zearalenone | 6,1 ± 0.07 | 7.8 ± 0.13 | 7.3 ± 0.15 | 7.9 ± 0.1 | 8.1 ± 0.18 | |
| T-2 toxin |
5.6 ± 0.07 c |
6.8 ± 0.02 c |
6.4 ± 0.16 c |
7.6 ± 0.16 | 8.0 ± 0.21 | |
| Zearalenone+T-2 |
5.4 ± 0.07 c |
7.1 ± 0.01 c |
7.3 ± 0.15 | 7.6 ± 0.16 | 8.1 ± 0.18 | |
| Sperm cells survival rate, hours | without toxins | 7.8 ± 0.13 | 7.8 ± 0.13 | 9.1 ± 0.1 | 8.2 ± 0.13 | 8.3 ± 0.15 |
| Zearalenone | 7.8 ± 0.13 | 7.8 ± 0.13 | 9.1 ± 0.1 | 8.2 ± 0.13 | 8.3 ± 0.15 | |
| T-2 toxin |
5.8 ± 0.13 c |
6.9 ± 0.1 c |
6.3 ± 0.15 c |
6.9 ± 0.1 c |
7.5 ± 0.22b |
|
| Zearalenone + T-2 |
5.0 ± 0.0 c |
6.2 ± 0.13 c |
6.8 ± 0.13 c |
6.8 ± 0.1 c |
7.6 ± 0.22 a |
|
| The absolute survival rate, conventional units | without toxins | 31.4 ± 0.07 | 40.4 ± 0.4 | 39.45 ± 0.43 | 40.4 ± 0.4 | 42.0 ± 0.45 |
| Zearalenone | 31.4 ± 0.07 | 40.4 ± 0.4 | 39.45 ± 0.43 | 40.4 ± 0.4 | 42.0 ± 0.45 | |
| T-2 toxin |
16.6 ± 0.07 c |
23.4 ± 0.27 c |
22.6 ± 0.12 c |
36.4 ± 0.3 c |
40.0 ± 0.52b |
|
| Zearalenone + T-2 |
15.5 ± 0.11 c |
20.2 ± 0.13 c |
25.5 ± 0.18 c |
35.9 ± 0.1 c |
40.4 ± 0.58 c |
|
| Indicators of the thawed sperm of bulls | ||||||
| Sperm cells activity (after one-hour exposure), points | without toxins | 3.8 ± 0.13 | 3.8 ± 0.13 | 3.9 ± 0.1 | 3.9 ± 0.1 | 4.1 ± 0.1 |
| Zearalenone |
3.1 ± 0.07 c |
3.7 ± 0.13 | 3.9 ± 0.1 | 3.9 ± 0.1 | 4.1 ± 0.1 | |
| T-2 toxin |
0.6 ± 0.07 c |
1.9 ± 0.07 c |
3.2 ± 0.13 c |
3.1 ± 0.1 c |
3.7 ± 0.15 a |
|
| Zearalenone + T-2 |
1.4 ± 0.07 c |
2.1 ± 0.07 c |
3.2 ± 0.13 c |
2.9 ± 0.02 c |
4.0 ± 0.15 | |
| Sperm cells survival rate, hours | without toxins | 5.2 ± 0.13 | 4.2 ± 0.13 | 5.1 ± 0.1 | 5.1 ± 0.1 | 5.2 ± 0.13 |
| Zearalenone |
3.2 ± 0.13 c |
3.4 ± 0.16 c |
4.8 ± 0.13 | 5.1 ± 0.1 | 5.2 ± 0.13 | |
| T-2 toxin |
1.4 ± 0.07 c |
1.6 ± 0.07 c |
4.6 ± 0.16 | 4.8 ± 0.1 |
4.8 ± 0.13a |
|
| Zearalenone + T-2 |
1.9 ± 0.07 c |
2.0 ± 0.0 c |
4.4 ± 0.16 |
4.6 ± 0.2c |
4.9 ± 0.1 | |
| The absolute survival rate, conventional units | without toxins | 12.6 ± 0.07 | 7.8 ± 0.13 | 15.1 ± 0.05 | 15.4 ± 0.07 | 16.9 ± 0.28 |
| Zearalenone |
6.4 ± 0.07 c |
6.8 ± 0.13 c |
13.1 ± 0.05 c |
12.1 ± 0.07 c |
16.9 ± 0.28 | |
| T-2 toxin |
1.6 ± 0.07 c |
2.4 ± 0.07 c |
12.4 ± 0.07c |
9.9 ± 0.1 c |
15.8 ± 0.33a |
|
| Zearalenone + T-2 |
1.8 ± 0.08 c |
3.6 ± 0.07 c |
12.4 ± 0.07 c |
8.4 ± 0.07 c |
16.5 ± 0.34 | |
Note: a, P< 0.05; b,– P < 0.01; c,– P < 0.001 (compared to the reference without toxins).
It has been found that upon the addition of 0.5 mM of Zearalenone to the diluent, the activity of native sperm cells did not change for one hour after the exposure, while after thawing, it decreased by 19.4 % compared to the original level, or by 0.69 points (P < 0.01), compared to the non-treated negative control. At the same time, the survival rate of native sperm reduced by 10.6 % (P<0.05), and for thawed sperm by 27.9 % (P < 0.001). The absolute survival rate of native spermatozoa reduced by 3.0 % (P < 0.01), and for the thawed ones by 46.47 % (P < 0.001). The T-2 toxin at the concentration of 0.5 mM reduced the activity of native sperm after one hour of incubation by 10.14 % (P < 0.01) while their activity after thawing decreased by 60.28 %, or 2.14 points (P < 0.001), compared to non-treated negative control. The survival rate of native sperm cells of stallions reduced by 10.6 % (P < 0.05), and for the thawed ones by 27.9 % (P < 0.001). Likewise, adding the T-2 toxin to the diluent contributed in decreasing the absolute survival rate of sperm cells by 29.5 % (P<0.001), and for thawed ones by 70.2 % (P < 0.001).
Upon the simultaneous addition of 0.5 mM of Zearalenone and T-2 toxin to the diluent, the activity of native sperm cells deteriorated after one hour of incubation by 15.64 % (P < 0.001), and the activity of the sperm cells after thawing decreased by 83.4 %, or 2.9 points (P < 0.001), compared to non-treated negative control. The native sperm survival rate decreased by 39.3 % (P < 0.001), and for thawed sperm by 72.1 % (P < 0.001). The absolute survival rate of native sperm decreased by 41.2 % (P<0.001), and for thawed sperm by 84.6 % (P < 0.001).
Zearalenone at the concentration of 0.25 mM in the diluent after one hour of incubation did not affect the activity of native spermatozoa; however, their activity after freezing and thawing deteriorated by 12.2 %, or 0.41 points (P < 0.05), compared to non-treated negative control. The survival rate of native sperm cells reduced by 3.54 %, and for thawed ones by 26.7 % (P < 0.001). The absolute index of native spermatozoa survival rate decreased by 3.0 % (P < 0.01), and for the thawed ones decreased by 45.9 % (P < 0.001). The T-2 toxin at the concentration of 0.25 mM reduced the activity of native sperm after one hour of exposure by 8.03 % (P < 0.05); the activity after thawing reduced by 52.7 % (P < 0.001), compared to decreased. The native sperm cells’ survival rate decreased by 22.8 % (P < 0.001), and for the thawed ones decreased by 52.3 % (P < 0.001). The absolute index of native spermatozoa survival rate deteriorated by 29.0 % (P < 0.001), and that of the thawed ones decreased by 69.4 % (P < 0.001). Upon the simultaneous addition of 0.25 mM of Zearalenone and the T-2 toxin, the activity of native sperm after one hour of exposure deteriorated by 11.12 % (P < 0.01), and the activity after thawing decreased by 77.1 %, or 2.59 points (P < 0.001), compared to decreased. Within that, the native sperm survival rate deteriorated by 38.61 % (P < 0.001), and that of thawed sperm decreased by 71.15 % (P < 0.001). The absolute index of native spermatozoa survival rate decreased by 40.98 % (P < 0.001), and that of the thawed ones by 82.84 % (P < 0.001).
Upon reduction of the dosage of Zearalenone to 0.1 mM, after one hour of exposure, the activity of native sperm did not deteriorate, and the activity of the sperm cells after thawing decreased by 14.4 % (P < 0.01) while the survival of native sperm reduced by 3.54 %, and for thawed sperm decreased by 15.1 % (P < 0.01). Also, the absolute survival rate reduced for native sperm by 1.82 %, and for thawed sperm decreased by 43.2 % (P < 0.001). After adding the T-2 toxin alone in the amount of 0.1 mM, a decrease by 7.78 % (P < 0.05) was observed in the activity of the native sperm after one hour of exposure, while the activity after thawing decreased by 54.71 % (P < 0.001), compared to non-treated negative control. After adding the T-2 toxin in the amount of 0.1 mM, the survival rate of native sperm reduced by 12.6 % (P<0.01), and for thawed sperm decreased by 42.34 % (P < 0.001); the absolute survival rate decreased by 25.8 % (P < 0.001), and for thawed decreased 67.6 % (P < 0.001). Simultaneous adding of Zearalenone and T-2 toxin to the diluent at the dosage of 0.1 mM had a more pronounced cytotoxic effect than each one separately and reduce the activity of native sperm after one hour of incubation by 12.4 % (P < 0.01), and the activity after thawing by 75.1 % (P < 0.001), while the survival rate of native sperm reduced by 30.3 % (P < 0.001), and that of thawed sperm decreased 66.3 % (P < 0.001); the absolute survival rate decreased by 38.3 % (P < 0.001), and that of thawed sperm by 78.8 % (P < 0.001).
Upon further decrease in toxins concentrations, lower tendency of their cytotoxic effect on the germ cells in stallions was recorded. In this study, the lowest concentration that yield a significant decrease in the sperm biological indicators of was 0.01 Mm that it had no cytotoxic impact on native spermatozoa of stallions, while after thawing only a slight decrease in the activity and survival rate of sperm cells was observed by 2.3 and 6.5 %, respectively. Meanwhile, at the dosage of 0.01 mM, a veraciously significant decrease in the survival rate and the absolute survival indicator of the native spermatozoa by 4.3 and 8.7 % (P < 0.05), respectively, that observed upon the simultaneous addition of Zearalenone and T-2 toxin.
Mycotoxins have strong cytotoxic action on the germ cells of males, as evidenced not only by the large number of the spermatozoa plasma membrane damages, but also by the damages to the anatomical section of the “neck” of sperm cells in stallions, which is probably the reason for decreasing the complex of spermatozoa biological parameters after thawing. Figure 1 shows a four to five times increase in the amount of sperm cells’ membranes damage under the action of Zearalenone and T-2 toxin. Besides, Zearalenone and T-2 toxin also cause irregularities in the morphology of the acrosomes of sperm cells in bulls (Figure 2).
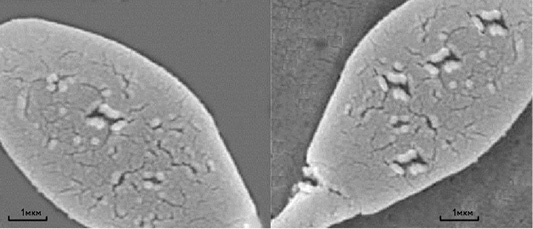
Figure 1: Scanning electron microscopy of thawed sperm cells of a stallion without the addition of mycotoxin (left) and with the addition of mycotoxin (right). Scale segments - 1 µm.
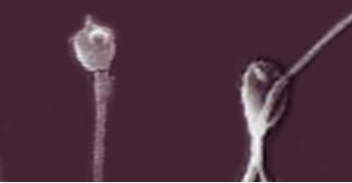
Figure 2: Light microscopy of thawed bull sperm cells with the addition of Zearalenone and the T-2 toxin (sperm cells with damaged acrosomes are shown). Magnification 100×.
Figure 2 shows the destruction of the acrosomes of spermatozoa in bulls (left and right) under the action of Zearalenone and T-2 toxin with the appearance of pathological forms of sperm cells in the form of increased brittleness of the tails (right). The determined ability of mycotoxins to destroy acrosomes of sperm cells may reduce the fertilizing ability of sperm during IVF (in vitro fertilization) in humans and artificial insemination in animals due to the decreased level of hyaluronidase, which is required for the process of fertilization.
For determining the cytotoxic effect of the studied mycotoxins on the spermatozoa of bulls, the tendency concerning the studied basic biological parameters of the spermatozoa was preserved as shown in Table 2 that showed that with preservation, the overall tendency of the cytotoxic effect of Zearalenone and the T-2 toxin on the biological indicators of the sperm cells of bulls. It is also evident that after thawing, the T-2 toxin alone was more toxic than the simultaneous effect of Zearalenone and the T-2 toxin.
For the successful use of embryo transplanting to donor cows, the introduction of high-quality sperm is required in addition to sticking to the technology of feeding and housing (Baruselli et al., 2010). Based on previous studies and practice experience, show that the use of high-quality sperm significantly increases the percentage of fertilization, both in vitro and in vivo. The sperm quality should be assessed at all stages of handling; from obtaining from the stud bull to the use in animal breeding. At animal breeding farms, artificial insemination of donor cows with poor quality sperm, the index of cow insemination will be reduced along with the fertility after the first insemination and, consequently, the service period will be shortening.
At the station for black-motley cattle artificial insemination (LLC Agricultural Farm Mikhailovskoe, the Kemerovo region), only the activity of the sperm cells is assessed. To check the quality of the sperm stored at −196 °C, the Dewar’s vessel is opened, the sperm is thawed, and sperm cell mobility is examined under a microscope. The sample is taken quickly to prevent the temperature shock of the sperm. The sperm is examined at 38–40 °C with using of heating table. If the activity of bull sperm is less than three points, the sperm is not recommended to be used for fertilization. The thawed sperm cannot be frozen again. The mobility of the sperm cells in thawed uncoated granules is determined the same as in the sperm stored at 2–5 °C. The shell of the packing of semen dosages in coated granules after thawing is wiped with gauze, the edge of the packing is clamped between two glass slides using special spring clips or clothes pegs, put on the heating table of the microscope at low magnification, and the sperm is viewed through film packing. Thawed sperm with the mobility not lower than 3 – 4 points is allowed to be used for cows and heifers.
Since sperm is purchased and delivered at the breeding farm, all of its parameters should already be checked according to GOST 8607-2015. However, in the case of improper feeding, mycotoxins can accumulate in the embryos even before washing off, which affects fertility. Therefore, upon receipt of frozen semen, quantitative microbiological monitoring of the hygiene and processing of the sperm of bulls to meet the biosafety requirements for efficient artificial insemination of donor cows. For this reason, it is very important to study the bacterial contamination and the possible presence of facultative pathogens in the frozen sperm of bulls. Therefore, it is important to check the bacterial contamination for sperms according to the above-mentioned scheme, before introducing it to donor cows.
DISCUSSION
The current study aims to show the differences between the cytotoxic effect of Zearalenone and T-2 toxin on the main biological indicators of the sperm of bulls and stallions after cryopreservation. The practical need for such a study is due to a sharp decrease in the efficiency of sperm cryopreservation either in animals and in humans (Francavilla et al., 2003). Previous studies reported that sperm of 50 % of the stallions in the world cannot survive after freezing and thawing (Graham, 1996; Katila, 2001; Mottershead, 2000; Tkachev, 2013a). Based on the foregoing, we studied the cytotoxic effect of mycotoxins in the feed on increasing the chromosomal aberrations in somatic cells of stallions. It was reported that the long-term feeding of horses on the feed with even acceptable levels of mycotoxins significantly decreased the content of testosterone and increased the content of estradiol in the blood of stallions (Tkachev, 2014a). Our hypothesis suggest that the efficiency of animal sperm cryopreservation might depend on Zearalenone and T-2 toxin that were most often present in the animal feed. It has been widely known that the sperm survival rate indirectly depends on the level of testosterone in the blood (Filannino et al., 2011). The less is testosterone, the less sugar is in the sperm, and lower survival rate and the fertilizing capacity for the spermatozoa (Filannino et al., 2011). Previous studies showed that adding Zearalenone and its derivatives to the freshly obtained stallion sperm increased its activity and decreased the survival rate in vitro, possibly due to more rapid consumption of nutrients; however, in this experiment, the exposure to the toxins did not exceed two hours (Filannino et al., 2011).
Other studies showed that mycotoxins gives with the fodder even within acceptable concentrations can be accumulated in the prostate, testes, ovaries, and even in the hypothalamus, interacting with the glucuronic acid (Gromadzka et al., 2008). Mycotoxins can reduce the initial characteristics of sperm in humans and animals, which subsequently may influence the efficiency of freezing and thawing for the sperm (López-Fernández et al., 2007). Presumably, this happens due to the destruction of the structure of the chromatin of the sperm cells (López-Fernández et al., 2007). Moreover, with the accumulation in the hypothalamus, mycotoxins can cause serious disruption of the hormonal regulation in the entire host (Diaz, 2006). Meanwhile, the possibility of urinal excretion of large amounts of mycotoxins (up to 7 mM) in horses reasonably allows assuming the possibility of their getting into the sperm during ejaculation (Erasmuson et al., 1994). Our results have convincingly proven the necessity of determining the minimum concentrations of mycotoxins, which would reduce the main biological indicators of the sperm of bulls and stallions during cryopreservation, since the fertility of animals and humans may decrease in the future.
The established fundamental difference of the cytotoxic effect of mycotoxins during cryopreservation was that, T-2 toxin alone was more toxic than the simultaneous addition of Zearalenone and T-2 toxin. While, simultaneous addition of Zearalenone and T-2 toxin into the diluent was more toxic to the sperm of horses than the addition of T-2 toxin alone. We assume that this may be mainly due to the difference in biochemical metabolism of stallion sperm (respiration-dominated) and bull sperm (glycolysis-dominated) with the prevalence of glycolysis and a greater number of sperm cells in the sperm of bulls (800 – 1,200 million sperm cells per cm3 against 100 – 200 million sperm cells per cm3 of ejaculate in stallions). There is no synergism between Zearalenone and T-2 toxin occurs. The obtained data suggest that the synergistic action of Zearalenone and the T-2 toxin is observed in the case of the predominance of the respiration processes in the presence of glycerol. Indirectly, this hypothesis is confirmed by previous literature data that describe the competitive binding of Zearalenone to the receptors of hormones of the cells (López-Fernández et al., 2007), and by the information that estrogen-like effect of Zearalenone may be associated with the increased activity of aromatase which transforms androgens into estrogens (Sanderson and van den Berg, 2003).
In this study, we succeed to prove for the first time that the cytotoxic properties of mycotoxins increase dramatically during cryopreservation of bulls and stallions’ spermatozoa in the form of severe damage to the membranes which might be due to low efficiency for freezing and thawing. At the same time, the destruction of the acrosome of sperm cells by mycotoxins deprives them of the important enzymes (hyaluronidase, acrosin) that are required for fertilizing the ovum in vitro and in vivo. We assume that this may also be related to the fact that Zearalenone and T-2 toxin in the presence of glycerol increase the intensity of lipid peroxidation by increasing the amount of diene conjugates and malondialdehyde, which damage the bi-lipidic layer of the plasmalemma. This hypothesis is based on the previous studies about increasing the content of diene conjugates and malondialdehyde in the blood of horses under the action of acceptable levels of mycotoxins in the fodder (Tkachev, 2014a; 2015a).
So, there is an urgent need to look into compounds that will be able to neutralize the cytotoxic effect of mycotoxins during cryopreservation of the sperm of animals and humans.
CONCLUSION
The cytotoxic effect of Zearalenone and T-2 toxin on the sperm of bulls and stallions in vitro after cryopreservation has been studied in vitro after cryopreservation. It has been found that the minimum cytotoxic dosage of Zearalenone and T-2 toxin during the cryopreservation was 0.01 mM. Likewise, the species differences have been shown in the sensitivity of the spermatozoa of bulls and stallions for the cytotoxic effect of Zearalenone and T-2 toxin during cryopreservation, which is evidence to the fact that complex addition of Zearalenone and T-2 toxin to the diluent has lower toxicity to the plasmalemma, acrosome, and the biological parameters of the spermatozoa of bulls than adding T-2 toxin alone, which is not observed in the sperm of stallions.
ACKNOWLEDGMENT
The article was prepared within the framework of Agreement with the Ministry of Education and Science of Russia No. 05.607.21.0208 “Development of a technology of genome editing for the reproduction of high-value pedigree dairy cattle resistant to the virus of leucosis”, unique identifier of agreement: RFMEFI60718X0208.
Authors Contribution
All authors contributed equally to the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





