Advances in Animal and Veterinary Sciences
Case Report
A Case Report of Wet Form Feline Infectious Peritonitis (FIP) in a Domestic Short Hair Cat
Abd Hamid Sifa-Shaida1, Abd Rahaman Yasmin1*, Saulol Hamid Nur-Fazila2, Raslan Ain Fatin2, Wan Noor Ayuni1, Zakaria Alif1, Hasliza Zuhir3
1Department of Veterinary Laboratory Diagnostics, 2Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. 3AZ Animal Clinic and Pet Hotel, 37 Jln Pendidik U1/31, Hicom-Glanmarie Industrial Park, 40150 Shah Alam, Selangor.
Abstract | Feline Infectious Peritonitis (FIP), a fatal disease of cat exists in two major forms namely effusive and noneffusive form. FIP is caused by mutated form of Feline Coronavirus (FCoV) classified under the genus of Alphacoronavirus. Despite common prevalence of FIP in Malaysia, further diagnosis remain challenging due to the complexity of the disease which often required multiple findings to confirm the disease. This case report highlights the progressive wet form of FIP in a male domestic short hair cat named Cromox presented to the Post Mortem Unit, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM). Manifestation of distended abdomen, icterus and flu was shown before the cat died. Post-mortem and histopathology analysis of affected organs were performed and since FIP was suspected, RT-PCR against polymerase gene of FCoV was carried out. The post mortem examination revealed generalised icterus at sclera, gums and integuments, straw colour peritoneal fluid and congestion of kidney and liver. Histopathology analysis showed infiltration of mononuclear cells in liver, pulmonary edema and renal desquamation. Meanwhile, RT-PCR and partial sequencing analysis showed evidence of positive Feline Coronavirus which was closely related to the FCoV from China and Netherland. Hence, the cause of death of Cromox was confirmed due to FIP infection. Only supportive treatment can be given to the FIP affected cat since the disease is usually fatal. Vaccination against FIP is not recommended and avoiding the sick cat to share litterbox in the multihousehold cat has been proven to be an effective way to prevent the occurence of FIP.
Keywords | Coronaviridae, Feline infectious peritonitis, Histopathology, Post-mortem, RT-PCR
Received | April 14, 2020; Accepted | July 18, 2020; Published | August 10, 2020
*Correspondence | Nor Yasmin Abd Rahaman, Department of Veterinary Laboratory Diagnostics, Faculty of Veterinary Medicine, Universiti Putra, Malaysia, 43400 UPM Serdang, Selangor, Malaysia; Email: noryasmin@upm.edu.my
Citation | Sifa-Shaida A-H, Yasmin A-R, Nur-Fazila S-H, Ain-Fatin R, Ayuni W-N, Alif Z, Zuhir H (2020). A case report of wet form feline infectious peritonitis (Fip) in a domestic short hair cat. Adv. Anim. Vet. Sci. 8(10): 1045-1049.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.10.1045.1049
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Abd-Hamid et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Coronaviridae is a large group of virus family that capable of infecting wide range of host causing Covid-19 in human, Feline Infectious Peritonitis in cat, gastroenteritis in dog and Infectious Bronchitis in chicken. It has substantial ability to mutate leading to difficulty in treating the affected host. Feline Infectious Peritonitis (FIP) is caused by Feline Coronavirus (FCoV) and it is highly fatal disease in domestic and feral cats (Addie et al., 2009). The virus belongs to the family Coronaviridae of Genus Alphacoronavirus. The virion structure is pleomorphic spherical and it consists of positive-sense, single stranded enveloped RNA with the genome size of approximately 30kb of non-segmented gene. FCoV is divided into two biotypes which includes feline enteric coronavirus (FECV) and feline infectious peritonitis virus (FIPV) (Addie et al., 2009; Licitra et al., 2013). Usually the infection with FECV appear unapparent or elicit as transient gastroenteritis meanwhile FIPV usually can either present as wet form or dry form and it can cause fatal illness to the hosts with or without showing any specific clinical signs (Sharif et al., 2010). Dry form of FIP resembles non-specific clinical signs such as blindness and CNS related illness while effusive or wet form of FIP usually presented as distended abdomen. A research conducted in 2004 revealed that 100% of cats in Malaysia had antibodies against FCoV regardless of age and gender when tested with dot blot ELISA test kit ImmunoComb (Arshad et al., 2004). Evidence of FCoV antigen via rt-PCR and sequencing analysis was showed in 4.76% (1/21) pet owned cat in a state of Malaysia in 2017 (Hoong et al., 2019). FCoV have the ability to mutate to FIP and cause death to the infected cats however, the incidence of FIP are relatively low (Licitra et al., 2013; Pederson, 2014a). Confirmation of FIP infection and to predict whether the virus could mutate to FIP remain challenging particularly in alive cats.
Case report
History
This case report highlighted the approaches used to final diagnosis of FIP following the death of a cat. Cromox, a 7-months-old male domestic shorthair cat with a complete vaccination and deworming status history was presented to a private veterinary clinic on 26th December 2018 with the complaints of jaundice, flu and distended abdomen. Medication given upon that time was Doxycycline 100mg (antibiotics), Bromhexine 8mg (mucolytics) and Chloramphenaramine (anti-histamine). On 6th Jan 2019, another medication was prescribed which was Samilyn (liver supplement). However, 2 days later, the cat died and it was sent to the post mortem laboratory in UPM for further investigation.
Post-mortem findings
The body condition score (BCS) of the cat was 2 out of 5. Upon post mortem, generalised icterus was observed and mostly evident at integument, mucous membrane, sclera and conjunctiva (Figure 1A and 1B). Grossly, there was accumulation of straw coloured effusion with gelatinous clots within the abdominal cavity (Figure 1C).
Histopathology
Histopathology examinations were performed on lung, liver and kidney samples. Microscopically, the alveolar space of the lung were filled with mild to moderate proteineaceous exudate with leucocytic cells infiltration as indicative of pulmonary edema (Figure 2A). The liver was markedly filled with necrotizing hepatocytes with numerous mononuclear cells infiltration (Figure 2B). In kidney, diffuse cell death of the tubular epithelium that characterized by shedding and desquamation of the cell fragments of the renal tubules into the lumen were observed (Figure 2C).
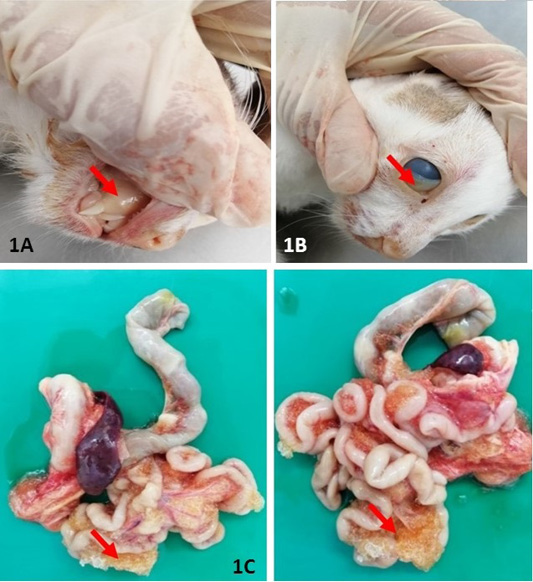
Figure 1: Gross lesion at necropsy: Extensive yellowish discolouration of mucous membrane (A) and conjunctiva (B) were indicative of severe jaundice. (C) and presence of extensive fibrinous plaque on serosal surfaces of intestines. All the lesions described have been indicated in arrow (↘).
Reverse-transcriptase PCR
RT-PCR targetting polymerase gene of FCoV was performed by using One Step RT-PCR (Bioline, MyTaq™ One Step) with the forward and reverse primer sequence, 5’ – GGT TGG GAC TAT CCT AAG TGT GA- 3’ and 5’ – CCA TCA TCA GAT AGA ATC ATC ATA- 3’, respectively to detect FCoV in lung, liver and kidney samples. Master mix was prepared and was transferred into each microcentrifuge tube. To visualize and purify the DNA fragments, gel electrophoresis was used to separate DNA according to their product size, length in base pairs. As a result, a band at 44s0 bp region aligned together with the positive control (Figure 3) indicative of positive FCoV was detected from the electrophoresis reaction of the PCR product of Cromox. The positive control used for PCR in the case was previously developed from a positive diagnostic case reported in UPM.
Partial sequencing analysis
The gel was cut and submitted for partial sequencing analysis. The result of BLAST showed that the sequence was 92-95% homologous as compared to the reference isolates found in the GenBank® of NCBI (JN183883.1, KY292377.1, FJ938053.1, FJ938054.1, KY566211.1, KY566210.1, KY566209.1, KF530123.1, FJ938059.1, FJ938056.1, FJ938055.1, MG893511.1, HQ392469.1, HQ012371.1, MH817484.1, HQ392472.1, HQ392471.1, KU215428.1, KU215427.1, KU215422.1, KU215421.1, KU215419.1). Phylogenetic tree was constructed and the reference isolates which is closest to sequence from Cromox were Feline Coronavirus strain HLJ/DQ/2016/01 complete genome (GenBank® accession no. KY292377.1) and Feline Coronavirus UU54 complete genome (GenBank® accession no. JN183883.1) (Figure 4) from China and Netherlands, respectively. Therefore, based on molecular analysis, definitive diagnosis of this case is wet form FIP.
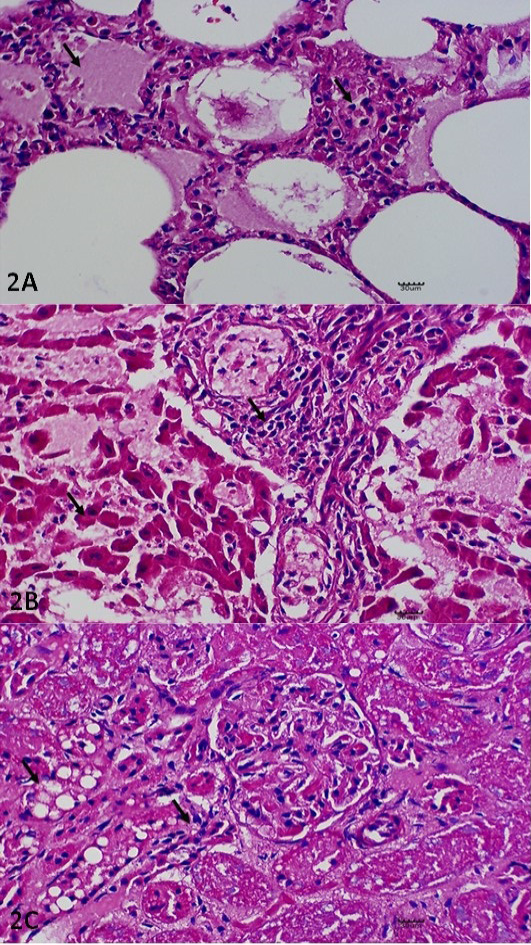
Figure 2: Histopathological Lesions. (A) Pulmonary edema and thickening of the interalveolar septae with leucocytic infiltration. H and E. X400. (B) Necrotising hepatitis, including necrotic changes on the hepatocytes with generalised infiltration of the leucocytes. H and E. X400. (C) Glomerular nephritis, including cytoplasmic vacuolation of the renal tubule (X) and epithelium clusters of scattered necrotic tubules (Y). H and E. X400. All the lesions described have been indicated in arrow (↘).
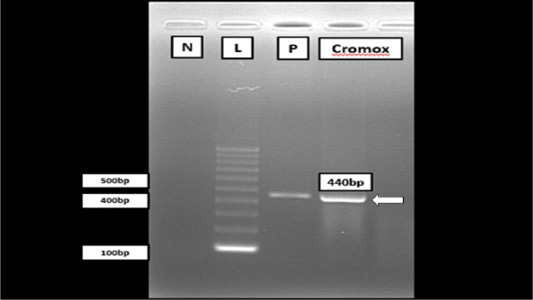
Figure 3: Gel electrophoresis of RT-PCR analysis from Cromox using primers targeting the conserved polymerase gene of FCoV. Lane N: Negative control; Lane L: 100 bp DNA marker (Vivantis, Malaysia); Lane P: Positive control; Lane C: Cromox. Remark: Deionized water was used as negative control.

Figure 4: The unrooted phylogenetic tree between the local isolates FCoV Malaysia Cromox (in text box) with the reference isolates. The phylogenetic tree was constructed using maximum-likelihood method with 1000 bootstrap replicates. Based on this phylogenetic tree, the strain of Cromox is closely related to strain found in China and Netherlands.
DISCUSSION
Under stressful environment such as multicat household and sharing of litter box with the infected cats with FECV, the likelihood of FECV mutation to FIPV is higher (Diaz and Poma, 2009). Many theories has been discussed on the mechanism of mutation, in which one of it is due to vigorous unprotective humoral immune response and causing the monocyte to get infected and transform FECV to FIPV (Cornelissen et al., 2007; Pedersen, 2009, 2014a). After the host is infected with FIPV, the infected monocyte subsequently release cytokines such as TNF-α and IL-1 which enhance the inflammatory response due to releasing of cytokines during the time of infection thus lead to vasculitis which increases the permeability of the cell which in return cause leakage of fluid into the peritonial cavity. When the circulating antigen-antibody complexes deposit in the endothelium of the blood vessels, the complement will be activated, therefore vasoactive agents will be released thus causing protein and fluid loss which leads to ascites or fluid accumulation inside the abdominal cavity (Gelberg, 2016). The role key in the immunopathological changes observed in FIP cases can be seen at the presence of infected monocyte and macrophages whereby these cells are the predominant inflammatory cells which can be detected either from pyogranulomatous lesions or effussions (Paltrinieri et al., 1999). As a results of apoptosis due to the inflammatory responses which induced tumor necrosis factor alpha (TNF-alpha) leads to decrease of the humoral immune response probably creating a form of escape from the body to detect the presence of viral antigens (Takano et al., 2007). Thus, in these reported case evidence of inflammation were seen in the vital organs such as lung, liver and kidney as shown in the histological findings. Therefore, the cause of death in Cromox case was due to failure of multiple organ due to hypoxia which cause reduced in cardiac output leading to low venous return caused by hypovolemic shock. According to Pederson (2014b), immunohistochemistry of the biopsied tissue is the gold standard for FIP diagnosis. Nevertheless, detection of FCoV antigen via RT-PCR can be used as FIP diagnosis as it has sensitivity and specificity of more than 90%, but the definitive diagnosis of FIP requires multiple tests due to the complexity of the diagnosis of FCoV. Detection of FCoV antigen in feces or tissues/exudate via RT-PCR can be used as FIP diagnosis in conjunction with other clinical symptoms. RT-PCR analysis of blood samples remains challenging as virus levels are often low, and avirulent virus may be present in blood without progression to feline infectious peritonitis (Sharif et al., 2011).
CONCLUSION
As a summary, a confirmatory diagnosis of wet form of FIP in this case was diagnosed based on the collective findings of the history and clinical signs coupled with findings of gross lesion, histopathological lesion, RT-PCR and sequencing analysis. In general, only supportive treatment can be given to the FIP affected cat as there is no specific treatment of FIP since the disease is usually fatal in cat. Vaccination againts FIP remain controversial to date due to the antibody enhancement phenomenon. Since the infection is usually fecal-oral, keeping the litter box as hygiene as possible, avoiding the sick cat to share the litterbox in the multihousehold cat and minimizing stress have been proven to be effective ways to prevent the occurence of FIP.
ACKNOWLEDGEMENTS
The authors would like to thank staff of Post mortem and Laboratory of Histopathology for technical assistance. This case report was supported by Universiti Putra Malaysia (UPM) under the research grants UPM 700-2/1/GP-IPM/2016/9510500 and UPM 700-2/1/GP-IPS/2017/9547800.
AUTHORS CONTRIBUTION
SSAH writing of the manuscript, sequence analysis. NYAR editing and reviewing of the manuscript, sequence analysis. AZ, NAFR and NFSH involved in necropsy, samples collection and histological interpretation. WNAWN conducted the PCR and sequencing. HZ veterinarian that attended the cat case before died. All the authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES





