Advances in Animal and Veterinary Sciences
Research Article
Improvement of Fermentation and thend the In Vitro Digestibility Characteristics of Agricultural Waste-Based Complete Feed Silage with Cellulase Enzyme Treatment
Budi Santoso*, Trisiwi Wahyu Widayati, Bambang Tjahyono Hariadi
Department of Animal Science, Faculty of Animal Science, University of Papua, Manokwari, West Papua 98314, Indonesia.
Abstract | The use of crop residues and agricultural wastes in animal feed is a very common practice to ensure a feed supply in livestock production. However, the agricultural wastes generally have high crude fiber content. The study aimed at establishing the effects of increasing cellulase levels on fermentation quality, chemical composition, and in vitro nutrient digestibility of complete feed silage containing oil palm frond and rice crop residue. The mixture of ingredients (king grass, rice crop residue, oil palm fronds, cassava waste, tofu waste, and lactic acid bacteria) were treated with (A) cellulase 0 mL/kg as control, (B) cellulase 1 mL/kg, (C) cellulase 2 mL/kg, (D) cellulase 3 mL/kg, and (E) cellulase 4 mL/kg. The initial lactic acid bacteria (LAB) concentration in inoculant was 6.6 × 105 cfu/g of fresh matter, and the cellulase enzyme used in the study was a commercial product (Novozymes ). Plastic silos containing approximately 500 g of silage material for each were stored at room temperature (28–30°C) for 30 days. The results of the study showed that adding cellulase increased crude protein content (L: P < 0.01), but decreased neutral detergent fiber (NDF) (L, Q: P < 0.01), acid detergent fiber, hemicellulose (L: P < 0.01), and cellulose (L: P < 0.05) contents. The silages with cellulase treatment had high concentrations of lactic acid, but low pHs and total volatile fatty acid VFA contents, which indicated that they had been successfully preserved. Adding cellulase improved (L: P < 0.01) in vitro digestibility of dry matter, organic matter, and NDF. The study showed that adding cellulase improved fermentation quality and in vitro nutrient digestibility of agricultural and food industry-wastes based complete feed silage.
Keywords | Cellulase, Fermentation, Oil palm frond, Rice crop residue, Silage
Received | January 19, 2020; Accepted | July 07, 2020; Published | July 20, 2020
*Correspondence | Budi Santoso, Department of Animal Science, Faculty of Animal Science, University of Papua, Manokwari, West Papua 98314, Indonesia; Email: b.santoso@unipa.ac.id
Citation | Santoso B, Widayati TW, Hariadi BT (2020). Improvement of fermentation and the in vitro digestibility characteristics of agricultural waste-based complete feed silage with cellulase enzyme treatment. Adv. Anim. Vet. Sci. 8(8): 873-881.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.8.873.881
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Santoso et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The use of crop residues and agricultural wastes in animal feed is a very common practice to ensure a feed supply in livestock production. Rice crop residues (RCRs) are the lower parts of the fresh rice crops after they have been harvested and usually left in field. Therefore, the residues are abundantly available. However, most of them are not used as livestock feeds and burned once they have been dry, which causes air pollution. Santoso et al. (2012) showed that RCRs could potentially be preserved as silage and used as ruminant feed. However, the byproduct has high fiber fractions, such as neutral detergent fiber (NDF) (71.3%) and acid detergent fiber (ADF) (46.5%).
Oil palm fronds (OPFs) represent byproducts of oil palm trees. Large amount of the OPFs are produced and could potentially be used as roughage sources for ruminants. In general, the pruning cycle of the OPFs is used to increase palm oil productivity. However, they also have large fiber fractions (79.2% NDF and 63.4% ADF) (Santoso et al., 2019).
One of the methods to improve the low-quality agricultural waste is to mix it with high-quality feed ingredients to produce complete feed silage. The complete feed system is increasingly appreciated as it extensively uses agro-industrial byproducts, crop residues, and non-conventional feeds in livestock rations, which helps maximize production and minimize feeding costs. The provision of the complete feed for beef cattle is economically more profitable than conventional feed (Mayulu et al., 2009) because complete silage technology can reduce feeding times and labor requirements (Baba et al., 2011). Maekawa et al. (2002) suggested that forage and concentrate feeding resulted individually in cows consuming higher proportion of concentrates than the expected consumption of the concentrates, which increases risk of ruminal acidosis (Beauchemin et al., 2002).
Ensilage has long been used to preserve forage crops for livestock. During fermentation process, epiphytic lactic acid bacteria (LAB) on forage crops uses water-soluble carbohydrates to produce lactic acid, which represents the primary acid responsible for decreasing the pH in silage (Pang et al., 2011). The addition of cellulase increases the amount of the substrates that are made available for the LAB after NDF degradation. The increase in the amount of the substrates could propagate the LAB during early ensiling stage (Sun et al., 2009). Furthermore, Ebrahimi et al. (2014) reported that addition of fibrolytic enzymes increased the availability of water-soluble carbohydrates (WSCs) resulting from fiber degradation. The WSCs can also support lactic acid bacteria proliferation. It can cause a significant increase in the lactic acid concentration followed by a decrease in pH value. Sheperd and Kung (1996) concluded that adding the enzymes to silage can produce more WSCs and improve the organic matter (OM) digestibility of the silage because they degrade plant cell walls. Ni et al. (2014) also reported that wheat straw silage with cellulase treatment had lower ADF and NDF values than control silage. Therefore, objective of study was to establish the effects of increasing the cellulase levels in the complete feed silage containing RCR and OPF on chemical composition, fermentation quality, and in vitro nutrient digestibility of the silage.
MATERIALS AND METHODS
Forage materials
Fresh OPF and RCR were obtained from local palm oil plantation and paddy field in Prafi District, Manokwari Regency, Indonesia. The location is situated at approximately 133°48’E and 00°53’S, at an average altitude of 128 m. Cassava and tofu wastes were respectively obtained from a small-scale food industry in Prafi and Manokwari Districts. Complete feed silage was prepared by drying the tofu and cassava wastes in a forced-air oven at least for 48 h at 60°C. And then, the resulting product was ground in a Wiley mill (ZM200, Retch GmbH and Co. KG, Haan, Germany) so that it passed through a 1 mm sieve. King grass (Pennisetum purpureophoides) was harvested in 50 days of regrowth after defoliation from the experimental field of the Faculty of Animal Science, University of Papua, Manokwari, Indonesia (134°04′E and 00°48′S with an average altitude of 110 m). The king grass, RCR, and OPF were chopped into pieces of approximately 1-2 cm of length, well mixed and moved to laboratory.
Inoculant preparation
Epiphytic LAB inoculant was prepared following Bureenok et al. (2006) as previously described by Santoso et al. (2015) and Santoso et al. (2019). Two hundred twenty grams of fresh king grass was macerated in 1000 mL of distilled water for 4 minutes using a blender. The macerate was filtered through double layers of cheesecloth and 600 mL of filtrate was blended with 18 g of glucose. The filtrate was thoroughly mixed and incubated anaerobically at 30°C for 2 days. And then, the inoculants were used as LAB source. The number of LAB individuals in the inoculants was counted before the experiment was carried out by adding the inoculants to de Man Rogosa and Sharpe medium (MRS) and incubating them at 35°C for 3 days (Bureenok et al., 2006).
Silage preparation and treatments
The chopped grass was thoroughly mixed with other ingredients (Table 1). And then, the resulting mixture was treated with (A) cellulase 0 mL/kg as control, (B) cellulase 1 mL/kg, (C) cellulose 2 mL/kg, (D) cellulase 3 mL/kg, and (E) cellulase 4 mL/kg. The cellulase enzyme used in the study was a commercial product (Novozymes ), and the initial LAB concentration in the inoculants was 6.6 × 105 cfu/g of fresh matter. Silage was ensiled in plastic silos. Each of the plastic silos contained approximately 500 g (fresh weight). The silos were tied using a plastic strap and stored at room temperature (28°C to 30°C). After 30 days of ensiling, they were opened and the chemical composition, fermentation quality, and in vitro digestibility of silage were analyzed.
Table 1: Composition of ingredients (%) of agricultural wastes based-complete feed silage.
| Experimental complete feed silage | |||||
| A | B | C | D | E | |
| King grass | 50 | 50 | 50 | 50 | 50 |
| Rice crop residue | 10 | 10 | 10 | 10 | 10 |
| Oil palm frond | 10 | 10 | 10 | 10 | 10 |
| Cassava waste | 12 | 12 | 12 | 12 | 12 |
| Tofu waste | 15 | 15 | 15 | 15 | 15 |
| L. plantarum | 3 | 3 | 3 | 3 | 3 |
| Cellulase (ml/kg) | 0 | 1 | 2 | 3 | 4 |
Preparation and analyses of the samples
Fresh silage samples were drawn after 30 ensiling days, dried in a forced-air oven at least for 48 hours at 60°C and then ground using a Wiley mill that they passed through a 1 mm screen. AOAC procedure (2005) was used to determine dry matter (DM), ash, and crude protein (CP). NDF, acid detergent fiber (ADF), and acid detergent lignin (ADL) concentrations were analyzed following Van Soest et al. (1991). Alpha-amylase and sodium sulfite were discarded from NDF concentration analysis.
Procedure to determine fermentation quality was as follows: 20 g of fresh silage was macerated with 70 mL of distilled water and stored in a refrigerator at 4 °C for 24 hours. The sample was then homogenized using a shaker for 15 minutes and filtered through four layers of cheesecloth. Subsequently, the filtrate was collected for analysis of pH, lactic acid, NH3-N and VFAs. The pH of silage was determined using a pH meter (Hanna Hi 9025, Hanna Instruments Italia Srl, Villafrance Padovana, Italy). The VFAs concentration were separated and quantified using a gas chromatograph (Varian CP-9002 GC, 1500 mm × 3 mm i.d.; column temperature, 130oC; detector temperature, 220°C; nitrogen pressure, 1.25 kg/cm2; Shimadzu Co., Japan). The lactic acid and NH3-N concentrations were respectively determined following Barker and Summerson (1941) and Chaney and Marbach (1962). The Fleigh point for the assessment of silage quality was calculated using the formula used by Ozturk et al. (2006) as follows: Fleigh point = 220 + (2 × DM% – 15) – (40 × pH), where Fleigh point values between 85 and 100 represent very good quality; 60–80, good quality; 55–60, moderate quality; 25–40, satisfying quality; and < 20, worthless.
In vitro rumen fermentation characteristics
Ruminal fermentation of complete feed silage samples was conducted using an in vitro gas production technique described by Menke and Steingass (1988), as demonstrated previously by Santoso et al. (2019). Briefly, 300 ± 5 mg of the oven-dried samples were put into 100 mL glass syringes (3 replications per sample) (Model Fortune, Häberle Labortechnik, Germany). Syringes were filled with 30 ± 1.0 mL of rumen fluid-buffer mixture and placed in a water bath at 39°C for 48 hours. Each syringe was then gently shaken by hand every 8 hours. The gas production from each syringe was recorded before incubation (0 h) and after 1, 2, 4, 6, 8, 12, 24, 36, and 48 hours of incubation.
The sample of about 10 mL of the syringe content was drawn at the end of the incubation period. The pH of the medium was immediately measured using a digital pH meter (Hanna, Hi 8520, Ronchi di Villafranca, Italy). Subsequently, the VFAs were determined by pipetting 0.2 mL of sub-sample into 1.5 mL micro centrifuge tubes containing 1 mL of 25 g/100 mL (w/v) metaphosphoric acid and then centrifuging them at 9000 × g for 10 minutes. Two milliliters of sub-sample were added to 2 mL of 20 g/L (w/v) NaCl for NH3-N analysis.
In vitro nutrient digestibility
The in vitro DM, OM, and NDF digestibilities were measured using a two-stage technique described by Tilley and Terry (1963) and previously used by Santoso et al. (2019). In brief, 250 mg of dry sample were put into glass tube and weighed. It was done in triplicate. Rumen fluid sample was drawn from two Ongole-cross breed cattle using a stomach tube sucker before morning feeding and filtered through four layers of cheesecloth into a pre-warmed thermos flask. Tubes were filled with 25 mL of rumen fluid-buffer mixture at 1:4 (v/v) ratio, gassed with CO2 and tightly sealed with corks. Three blanks containing 25 mL rumen fluid-buffer mixture only were included in each run. Tubes were incubated in a water bath at 39°C for 48 hours, followed by hydrolysis with a pepsin-HCl solution for another 48 hours. Finally, the residues were filtered through pre-weighed Gooch crucibles and dried at 105°C for 24 hours. Percentage of weight loss was determined and presented as in vitro DM digestibility (IVDMD) and in vitro NDF digestibility (IVNDFD). The remaining residue was ashed at 550 °C to determine in vitro OM digestibility (IVOMD).
Statistical analysis
The data of chemical composition, fermentation products and in vitro digestibility of the 30-day silages were analyzed using one-way variance analysis with general linear model procedure in SAS 9.1 for Windows (SAS Institute, 2002, Cary, NC, USA). Orthogonal polynomial contrasts were used to assess linear, quadratic, and cubic responses to cellulase levels. Significance was set at P < 0.05 and P < 0.01.
RESULTS AND DISCUSSION
The characteristics of the fermented grass extract
The number of LAB in the fermented king grass extracts increased by 28% and there was a subsequent decrease in pH from 6.39 to 3.72 after 48 hours of anaerobic incubation (Table 2). These results indicated that during anaerobic incubation, the LAB used WSC to produce lactic acid. The finding was in agreement with previous study conducted by Santoso et al. (2019), which showed that after 48 hours of incubation at 30 °C, the pH value of the king grass extract decreased from an average of 5.93 to 3.21. Similar changes in the pH value after 48 hours of incubation were also reported in previous studies, such as Bureenok et al. (2006) who used fermented juice epiphytic lactic acid bacteria (FLJB) made of napiergrass, and Wang et al. (2009) who used FJLB made of alfalfa.
Table 2: Changes of pH value and lactic acid number in king grass extract before and after 48 h of incubation.
| Before incubation | After incubation | |
| pH | 6.39 | 3.72 |
|
LAB (× 105 cfu/g) |
3.5 | 6.6 |
The physical characteristics and the chemical composition of the silages
Brownish green color resembling the color of the original silage material, acid smell, and lack of fungus were generally observed in all of the agricultural and food industry wastes-based complete feed silage. Table 3 summarized the chemical composition of the agricultural and food industry wastes-based complete feed silages after 30 days of fermentation. The dry matter and the OM contents of the silages were not affected (P > 0.05) by cellulase levels. During ensiling, the DM played a significant role in the fermentation process. Lactic acid bacteria growth decreased as the moisture content increased, and the rate and the extent of the fermentation decreased when the moisture content was greater than 70% (Sarnklong et al., 2010). The mean DM content of the silages was 40.3%, which was above that recommended by McDonald et al. (1991) and Chamberlain and Wilkinson (1996) for good silage (30%). However, the DM content in the study was similar to that reported by Santoso et al. (2019) (40.4%) for complete silage made of agricultural and food industry wastes. The organic matter content in all of the silage samples (92.7%) was in agricultural and food industry similar to the OM content of the waste-based complete silage reported by Santoso et al. (2019).
Table 3: Chemical composition (%) of agricultural wastes-based complete feed silage with cellulose addition after 30 days of ensilage.
| Complete feed silages | SEM | P-value | |||||||
| A | B | C | D | E | L | Q | C | ||
| Dry matter | 40.6 | 40.0 | 40.2 | 40.0 | 40.7 | 1.76 | 0.95 | 0.76 | 0.97 |
| Organic matter | 92.9 | 93.6 | 92.93 | 93.4 | 92.8 | 0.33 | 0.78 | 0.30 | 0.81 |
| Crude protein | 13.4 | 14.6 | 14.1 | 14.4 | 15.9 | 0.29 | <0.01 | 0.15 | 0.01 |
| NDF | 50.0 | 48.1 | 46.2 | 44.8 | 42.1 | 0.44 | <0.01 | 0.01 | 0.01 |
| ADF | 37.9 | 34.7 | 33.2 | 32.8 | 31.1 | 0.51 | <0.01 | 0.06 | 0.10 |
| Hemicellulose | 15.1 | 13.4 | 13.0 | 12.0 | 11.1 | 0.46 | <0.01 | 0.56 | 0.39 |
| Cellulose | 23.3 | 22.4 | 21.8 | 21.6 | 21.3 | 0.59 | <0.01 | 0.52 | 0.85 |
SEM: standard error of the mean; L: linear effect; Q: quartic effect; C: cubic effect;No quartic polynomial was significant.
The CP concentration of the silages increased (L, C: P < 0.01) as the cellulase levels rose. The finding was consistent with previous study by Ebrahimi et al. (2014) and He et al. (2018) who added cellulase to oil palm frond silage and Neolamarckia cadamba leaf silage, respectively. They reported that the CP content significantly increased by 7.2% and 7.7%, respectively. Furthermore, the observed increases in the CPs of the ensiled OPF were most likely to relate to the decrease in NDF and, to a lesser extent, the ether extract content of the silage. Another causal factor of the increase in protein content with increasing cellulase levels could be low protein degradation levels. Oladosu et al. (2016) reported that proteolytic (putrefactive) enzyme types attacked amino acids and proteins. Their activities involved deamination (where ammonia was freed from amino acid), decarboxylation (the formation of amines), and oxidation/reduction (where amino acid was oxidized or reduced to form organic residues). Silage that underwent clostridial fermentation was characterized by high pHs, ammonia, butyric acid, and amine contents. The low silage pH of silage E probably inhibited the degradation of proteins by clostridia. Furthermore, the crude protein content of the silages in the study varied from 13.4 to 15.0%, which was higher than the recommended minimum protein content of 10–12% for ruminant diets (ARC, 1980).
Cellulase addition to complete silage significantly decreased NDF (L, C: P < 0.01), ADF (L: P < 0.01), and hemicellulose contents (L: P < 0.01), which indicated that the cellulose addition effectively degraded the cell wall carbohydrates. The finding corroborated Ebrahimi et al. (2014) and Ni et al. (2014) who showed that the cellulase addition alone or in combination with LAB caused a significant decrease in the NDF and ADF contents of ensiled OPF and wheat straw. The causal factor of it could be that the addition of cellulase stimulated the conversion of cellulose to WSC and hence rendered the available glucose available into LAB and also potentially converted it to lactic acid. Furthermore, Sun et al. (2012) showed that the cellulose addition increased the amount of NDF degrading substrates that could be used by the LAB. It meant that the propagation of the LAB could potentially be promoted during the early stage of ensiling, which would cause the rapid increase in lactic acid and the decrease in pH and hence inhibited the activity of harmful bacteria and proteolytic plant enzymes. The NDF improvement and ADF degradation related to the fact that the cellulase was also supported by the increase in the in vitro digestion of DM, OM, and NDF as presented in Table 5.
The fermentation quality of the complete feed silage
Variables, such as pH value, VFAs, and NH3-N concentrations, are commonly used as criteria to assess the quality of silage fermentation. The fermentation quality of the complete silages with cellulase treatment after 30 days of fermentation is shown in Table 4. The silages with the cellulase treatment had lower pH value (L, Q: P < 0.01) and higher lactic acid concentration (L, C: P < 0.01). Silage pH is one of main factors that influence the extent of fermentation and the silage quality of ensiled forage because low pH ensures that the forage remains in a stable form. The results showed that the silage pH decreased with increasing cellulase levels and it was consistent with previous study by Ebrahimi et al. (2014) and Ni et al. (2014). In this study, the pH values of the silages with the cellulase addition were in the normal pH range of 4.0 to 4.5 as recommended by Chamberlain and Wilkinson (1996). The low pH value of the silage with the cellulase addition (B, C, D and E) resulted from the high VFA concentrations in the treatments. Danner et al. (2003) reported that lactic acid was more effective in lowering silage pH than other VFAs and its concentration was higher than other acids.
Table 4: Fermentation characteristics of agricultural wastes-based complete feed silage with cellulose addition after 30 days of ensilage.
| Complete feed silages | SEM | P-value | ||||||||
| A | B | C | D | E | L | Q | C | |||
| pH | 4.61 | 4.38 | 4.32 | 4.26 | 4.22 | 0.02 | <0.01 | <0.01 | 0.09 | |
| Lactic acid (g/kg DM) | 68.9 | 76.9 | 80.2 | 81.5 | 90.6 | 1.15 | <0.01 | 0.97 | <0.01 | |
|
Lactic acid bacteria (×106 cfu/g) |
1.9 | 5.5 | 5.5 | 5.6 | 7.7 | 3.03 | 0.24 | 0.82 | 0.59 | |
|
NH3-N (g/kg total N) |
95.9 | 95.8 | 90.8 | 89.4 | 88.3 | 8.44 | 0.35 | 0.95 | 0.82 | |
| Acetic acid (g/kg DM) | 9.2 | 8.8 | 7.8 | 7.0 | 7.1 | 0.31 | <0.01 | 0.76 | 0.84 | |
| Propionic acid (g/kg DM) | 0.77 | 1.10 | 1.4 | 1.0 | 1.8 | 0.31 | 0.07 | 0.91 | 0.27 | |
| Butyric acid (g/kg DM) | 0.3 | 0.3 | 0.4 | 0.5 | 0.6 | 0.09 | 0.04 | 0.58 | 0.86 | |
| Total VFA (g/kg DM) | 10.3 | 10.2 | 9.5 | 9.3 | 9.4 | 0.46 | 0.02 | 0.62 | 0.66 | |
| Lactic acid:Acetic acid | 7.5 | 8.8 | 10.3 | 10.4 | 12.8 | 0.26 | <0.01 | 0.38 | 0.03 | |
| Acetic acid:Total acid | 0.11 | 0.10 | 0.9 | 0.9 | 0.10 | 0.01 | <0.01 | 0.39 | 0.19 | |
| Total VFA:Total acids | 0.13 | 0.12 | 0.11 | 0.10 | 0.09 | 0.01 | 0.01 | 0.21 | 0.66 | |
| Fleigh Point | 81.9 | 89.4 | 92.7 | 94.7 | 94.7 | 3.35 | 0.01 | 0.40 | 0.66 | |
SEM: standard error of the mean; L: linear effect; Q: quartic effect; C: cubic effect; No quartic polynomial was significant.
Table 5: In vitro nutrient digestibility of agricultural wastes based-complete feed silage with cellulose addition after 30 days of ensilage.
| Complete feed silages | SEM | P-value | |||||||
| A | B | C | D | E | L | Q | C | ||
| IVDMD (%) | 67.5 | 69.2 | 69.9 | 71.5 | 73.2 | 0.74 | <0.01 | 0.72 | 0.67 |
| IVOMD (%) | 69.5 | 71.4 | 72.6 | 74.9 | 76.9 | 0.54 | <0.01 | 0.52 | 0.79 |
| IVNDFD (%) | 28.5 | 29.3 | 29.7 | 32.8 | 36.1 | 0.83 | <0.01 | 0.03 | 0.82 |
SEM: standard error of the mean; L: linear effect; Q: quartic effect; C: cubic effect; No quartic polynomial was significant.
The lactic acid concentrations in the silages B, C, D, and E were respectively higher (11.6%, 16.4%, 18.3%, and 31.5%) than the lactic acid concentration in the silage A (control). The increase in the lactic acid concentration coincided with the increase in cellulose levels. It could have been caused by the increased degradation of NDF and ADF by cellulolytic enzymes. The increased NDF and ADF levels may have provided a substrate that the LAB could use to produce lactic acid. The lactic acid concentrations in the silages C, D, and E varied from 80.3 to 90.6 g/kg DM, which were in the ideal concentration range of 80–120 g/kg DM reported by Chamberlain and Wilkinson (1996).
The LAB numbers were not affected (P > 0.05) by the increase in cellulase levels. Generally, when the LAB number reaches at least 105 (cfu/g of fresh matter), silage will be well preserved (Cai et al., 1999). Table 4 shows that the LAB numbers in all of the silages were more than 105 cfu/g. It showed that the LAB numbers were sufficient to maintain good fermentation during ensilage. Furthermore, there was not any effect (P > 0.05) on the NH3-N, propionic acid, butyric acid, and total VFA concentrations in the silage. The butyric acid and NH3-N concentrations are important indicators in determining the fermentation quality of silage because they usually relate to clostridia activity. Owens et al. (2002) reported that proteolytic clostridia had the biggest contribution to degradation of amino acids into ammonia and non-protein nitrogen fractions. It indicated that the NH3-N concentration was the best indicator of secondary fermentation. The NH3-N concentrations in all of the silages varied from 88.3 to 95.9 g/kg total N, which could almost be acceptable for preserved silage. It was consistent with Haigh (1995) who stated that NH3-N < 100 g/kg N usually indicated successful preservation.
The acetic acid and butyric acid concentrations decreased linearly (P < 0.01) when cellulase was added. The findings were consistent with previous study by Ebrahimi et al. (2014). They also corroborated Khota et al. (2017) who reported that addition of acremonium cellulase to sorghum silage increased acetic acid concentration. The acetic acid was produced in carbohydrate fermentation by enterobacteria. However, it was relatively minor end product, meaning that enterobacteria were not desirable during silage fermentation. The acetic acid: Total fermentation acid ratio in all of the silages varied from 0.07 to 0.12, which was considerably less than recommended maximum value of 0.20 (Lima et al., 2011). Chamberlain and Wilkinson (1996) pointed out that secondary fermentation occurs if insufficient acid is produced by primary fermentation to reduce the pH to the value below the critical level of about 4.5. The bacteria responsible for secondary fermentation are mainly clostridia. The bacteria may convert lactic acid to butyric acid. Also, they may degrade protein peptides and amino acids into amines and ammonia. McDonald et al. (1981) also reported that butyric acid is produced by saccharolytic clostridia, i.e. Clostridium butyricum.
Total VFA consisted of concentrations of acetic acid, propionic acid, butyric acid, and other acids. The VFA indicated inefficient fermentation during ensilage or secondary fermentation as clearly observed in the change of lactic acid into butyric acid, the degradation of amino acids into ammonia and the production of acetic acid from the carbon skeleton of amino acids (Chamberlain and Wilkinson, 1996). In this study, the total VFA decreased linearly (P < 0.05) as the cellulose levels increased. The results indicated that the cellulase addition to complete silage could improve the fermentation quality of the silage. Proportions of total VFA to total acid for the silages A, B, C, D, and E were 0.13, 0.12, 0.11, 0.10, and 0.09, respectively. It showed that the silage E was the most efficient followed by the silages D, C, B, and A. Chamberlain and Wilkinson (1996) concluded that the total VFA proportion in ideal silage was less than 0.2.
Fleigh point is a value commonly used to assess the characteristics of silage fermentation based on dry matter content and pH value. The Fleigh point increased linearly (P < 0.01) as the cellulase addition increased. In the classification by Ozturk et al. (2006), the silage A (without any cellulose addition) and the silages B, C, D, and E (with cellulose addition) were in good quality classification. The Fleigh points of all of the silages were similar to the values reported for the agricultural wastes-based complete feed silage, which varied from 87.3 to 113.0 (Santoso et al., 2019).
In vitro nutrients digestibility
Digestibility is one of the most important factors affecting forage intake and highly depends on the forage chemical composition, especially cell wall content (Huhtanen et al., 2007). Table 5 shows the in vitro DM, OM, and NDF digestibilities of the complete feed silages containing OPF and RCR after cellulase addition. The in vitro digestibility reflects the degree to which microorganisms digest substrates in an artificial environment where rumen conditions are simulated in a test tube. Adding cellulase to complete silage significantly increased in vitro DM (L: P < 0.01), OM (L: P < 0.01), and NDF (L: P < 0.01 and Q: P < 0.05), and the DM, OM and NDF contents rose with the increase in cellulase levels. The increase in the DM digestibility was consistent with the results reported by Ebrahimi et al. (2014) who found that in vitro digestibility of DM in OPF silage with the cellulase addition increased 8% and 10% as compared to the fresh OPF. Furthermore, the increased IVOMD in sorghum silage after the cellulose addition was consistent with previous study by Khota et al. (2017). Additionally, higher IVNDFD levels seemed to relate to the physical characteristics of the silage, especially the NDF and ADF contents. Low NDF and ADF contents of the silages resulted in the rapid increase in DM, OM, and NDF digestibility. The results confirmed that the cellulose addition to agricultural and food industry-based complete silage could improve in vitro nutrient digestibility.
In vitro ruminal fermentation
The ruminal pH value, concentrations of NH3-N and VFA, and production of gas after 48 hours of incubation are presented in Table 6. The cellulase addition did not have any significant (P > 0.05) effect on the pH value and the NH3-N concentration. The ruminal pH values obtained in all treatments varied from 6.67 to 6.73, which were in the normal pH range of 6.0 to 7.0 to support optimal rumen fibrolytic activity. It is known that the pH value is an important factor in the inhibition of the growth of fibrolytic bacteria at the pH less than 6.0 (Weimer, 1996). The results of the study showed that the pH value was above 6.0 representing the normal pH necessary for microbial synthesis (Russell et al., 1992).
In the rumen, proteins and other nitrogenous compounds are broken down into ammonia that microbes use in protein and peptide synthesis. The ammonia concentration in the rumen is indicative of a balance between the degradation of feed protein and the uptake of ammonia for microbial protein synthesis. The NH3-N concentration in the study ranged from 49.5 to 54.4 mg/dL, which was above the optimal concentration for rumen NH3-N required to maximize microbial protein synthesis, which is 8.5 to about 30 mg/dL (McDonald et al., 2012). In other study, Abdulrazak et al. (1997) reported that 5–8 mg/100 mL rumen liquor could be sufficient for fiber digestion. Therefore, the ammonia N levels of all of the silage treatments in the study were sufficient to ensure optimum microbial growth and fiber digestion.
The cellulase addition increased acetic acid (L: P < 0.01), propionic acid (L, Q: P < 0.01), butyric acid (L: P < 0.01), and total VFA (L, Q, C: P < 0.01) concentrations. The high acetic acid, propionic acid, and butyric acid concentrations because of the cellulase addition were consistent with previous studies of tropical forages, such as Gilardo et al. (2004). McCollum et al. (1985) reported that the concentration of propionic acid in the rumen is affected by the fermentation of soluble carbohydrate. Volatile fatty acids produced in the rumen in a microbial fermentation process, are the main energy source for ruminants (Bergman, 1990). Bannink et al. (2008) suggested that the type of fermented substrate, microbial population, and rumen environment influence the type of VFA produced in the rumen. In this study, the silage E had the highest VFA concentration, indicating that the energy produced during incubation was also higher. The total VFA concentration varied from 72.8 to 102.3 mM, which was still in the normal range of 70-150 mM to maintain the growth of rumen microbes (McDonald et al., 2012).
Table 6: In vitro fermentation characteristics in the supernatant after 48 h incubation of agricultural wastes based-complete feed silage with cellulose addition.
|
Complete feed silages |
SEM |
P-value |
||||||||
| A | B | C | D | E | L | Q | C | |||
| pH | 6.67 | 6.67 | 6.67 | 6.70 | 6.73 | 0.03 | 0.10 | 0.39 | 0.79 | |
|
NH3-N (mg/dl) |
49.8 | 52.1 | 54.3 | 54.4 | 49.5 | 3.37 | 0.88 | 0.22 | 0.66 | |
| Acetic acid (mM) | 52.3 | 55.9 | 61.9 | 61.4 | 71.5 | 1.27 | <0.01 | 0.21 | 0.06 | |
| Propionic acid (mM) | 12.5 | 12.5 | 11.7 | 13.4 | 19.7 | 1.11 | <0.01 | <0.01 | 0.15 | |
| Butyric acid (mM) | 8.0 | 8.5 | 9.6 | 10.2 | 11.1 | 0.34 | <0.01 | 0.82 | 0.83 | |
| Total VFA (mM) | 72.8 | 77.0 | 83.3 | 84.9 | 102.3 | 1.23 | <0.01 | <0.01 | <0.01 | |
| Gas production (ml) | 67.3 | 68.5 | 73.8 | 77.2 | 79.2 | 1.23 | <0.01 | 0.92 | 0.19 | |
SEM: standard error of the mean; L: linear effect; Q: quartic effect; C: cubic effect; No quartic polynomial was significant.
The measurement of in vitro gas production is a good indicator to predict rumen degradability efficiency and metabolizable energy of animal feed (Contreras-Govea et al., 2011). The gas production pattern of the complete feed silage made of agricultural and a food industry waste in 48 hours is illustrated in Figure 1. The gas production in the 48 hours of incubation period increased linearly (L: P < 0.01) as the cellulase levels increased. The increase in gas production coincided with the decreases in the contents of the fiber fractions, such as NDF, ADF, hemicellulose, and cellulose (Table 2), which improved the digestibility of organic silage (Table 5). Beuvink and Spoelstra (1992) showed that OM digestibility, VFA concentration, and gas production have a significant correlation. The results of the study are consistent with Elghandour et al. (2015) who reported that the use of cellulase or/and xylanase at the dose of 1 µL/g DM of maize silage substrate improved in vitro rumen gas kinetics and cumulative gas production. They also indicate that the cellulase addition to complete silage increases the feed degradation efficiency in the rumen.
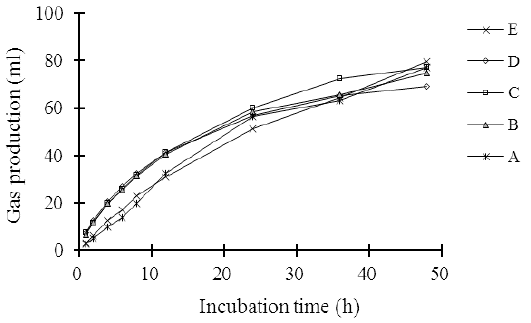
Figure 1: The pattern on in vitro gas production of agricultural wastes-based complete feed silage with increasing levels of cellulose.
CONCLUSION
The results of the study showed that cellulase addition increased CP content, but decreased fiber fractions, such as NDF, ADF, hemicellulose, and cellulose. The high lactic acid levels, and the low pH, and total VFA levels in the silages with the cellulase treatment indicated that they had been successfully preserved. They also indicated that the agriculture and industry wastes-based complete feed silage with the cellulase treatment improved the fermentation characteristics and the in vitro digestibility of nutrient.
ACKNOWLEDGEMENTS
The research was funded by The Directorate General of Strengthening Research and Development, Ministry of Research, Technology, and Higher Education of Indonesia under the Penelitian Terapan scheme with contract No. 198/SP2H/LT/DRPM/2019. The authors would like to thanks Zulkam Nurfahtorohman for his valuable assistance in sampling and preparation.
AUTHORs CONTRIBUTIONS
Budi Santoso developed the concepts, designed and performed the experiments, and drafted the manuscript. Trisiwi Wahyu Widayati and Bambang Tjahyono Hariadi performed the experiments, analyzed the samples, and performed the statistical analyses. All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
REFERENCES





