Advances in Animal and Veterinary Sciences
Research Article
Biochemical Quality Indices of Blue Swimmer Crab (Callinectes sapidus) Meat
Haitham Mohamed Elsayed1*, Fawzy Ramadan Hassanien2, Sayed Mekawy Ibrahim1, Abd El-Rahman Mohamed Sulieman2
1Fish Processing and Technology Laboratory, National Institute of Oceanography and Fisheries, Egypt; 2Food Science and Technology Department, Faculty Agriculture, Zagazig Univ., Egypt.
Abstract | This study was planned to investigate the proximate composition, quality indices, amino acids, fatty acids, volatile aromatic compounds and minerals content of blue swimmer crab meat. Blue crab (Callinectes sapidus) samples (average weight was 140.13±0.13g) were obtained from Baltim fish market, Kafar Elshaikh, Egypt during July, (2019). Blue crab meat samples were stored -18°C for 12 hrs. They were washed with tap water, manually dis-carapaces, then meat obtained was rewashed carefully and drained. The obtained results showed that the major constituents (wet wt.) of raw crab meat were 83.59% moisture, 9.12% crude protein, 1.80% lipid, 1.22% ash and 4.27% carbohydrates content. In addition, values of quality criteria of carp flesh were 7.35 pH, 47.42 mg/100g total volatile basic nitrogen (TVB-N), 0.19 mg/100g trimethylamine nitrogen (TMA-N) and 0.15 mg Malonaldhyed/kg sample thiobarbituric acid (TBA). Also, crab meat contained 19 volatile aromatics compounds, 8 essential amino acids (EAA), 9 nonessential amino acids (NEAA), 8 saturated fatty acids (SFAs) and 7 unsaturated fatty acids (USFAS). In addition, it contained high levels of Na, K, Ca and Mg. In general, this study confirms that blue crab meat is an important source of valuable nutrients AAS, FAs and major elements.
Keywords | Blue crab, Quality indices, Nutritive value
Received | April 23, 2020; Accepted | June 22, 2020; Published | July 20, 2020
*Correspondence | Haitham M. Elsayed, Fish Processing and Technology Laboratory, National Institute of Oceanography and Fisheries, Egypt; Email: haitham_m7716@yahoo.com
Citation | Elsayed HM, Hassanien FR, Ibrahim SM, Sulieman AM (2020). Biochemical quality indices of blue swimmer crab (Callinectes sapidus) meat. Adv. Anim. Vet. Sci. 8(8): 861-867.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.8.861.867
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Elsayed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Seafood is an important source of valuable nutrients, like minerals (e.g. calcium, iron, zinc, iodine, selenium, copper), vitamins, fatty acids (e.g. long chain n-3 poly-unsaturated fatty acids) and high quality proteins with essential amino acids, and is low in saturated fats (Nesheim and Yaktine, 2007). Some species of crabs are edible and number of others is commercially important for fishmeal industry. The crabs are increasingly realized as a potential food source for their delicacy and nutritional richness (Ravichandran et al., 2012). Moisture, crude protein, lipid and ash content were ranged from 53.56 to 89.4%, 8.02 to 15.7%, 0.23 to 0.97% and 0.35 to 6.22%, respectively as reported by Elegbede and Fashina-Bombata (2008); Marques et al. (2010); Özden and Erkan (2011); Varadharajan and Soundarapandian (2014); Silambarasan et al. (2016); Premarathna et al. (2015) and Yogesh Kumar et al. (2019). While pH, TVB-N and TBA values were ranged from 6.16 to 8.50; 7.85 to 19.29 mg/100g and 0.25 to 0.38 MDA/kg sample, respectively as found by Ibrahim, (2010); Bello, (2013); Shimaa et al. (2014) and AbouZeed (2016). Also, total EAA and NAA were 121.29 and 241.99 mg\100g, respectively as reported by Chen et al. (2013); Fulton and Elizabeth (2014); Kathirvel et al. (2014); Lu et al. (2014); Silambarasan et al. (2016) and Moruf and Lawal-Are (2019). While values of Oleic (c18:1), Linoleic (c18:2) and Stearic (c18:0) were 35.28, 57.71 and 40.23 mg\100g, respectively. As well as total aldehydes, aromatics, N-containing compounds, ketones and hydrocarbon compounds values were 1637.67, 140.36, 489.41, ND and 45.49, respectively of male chinese mitten crab (Eriocheir sinensis) as reported by Gu et al., (2013). And also, Sodium, Potassium, Calcium, Magnesium and Zinc content were 102.8, 136.4, 216.8, 79.5 and 9.84 mg\100g, respectively as reported by Silambarasan et al. (2016). In Egypt, total production of crab during 2017 was estimated about 5859 tons (3.21% of total catch 1.822.800 tons) as reported by the Generally Authority of Fish Resources Development (GAFRD, 2017). Therefore, this work aims to determine the biochemical analysis of raw blue crab meat.
MATERIALS AND METHODS
Crab samples
About 25 kg of raw blue crab (Callinectes sapidus Rathbun, 1896) samples were purchased from Baltim Fish Market, Kafar Elshaikh, Egypt during July, 2019. The blue crab was transported immediately using ice box within 5 hours to Fish Processing and Technology Laboratory, Elqanater Elkhairia Fish Research Station, National Institute of Oceanography and Fisheries. The average weight (Mean ± SD) of blue crab was 140.13±0.13g. Crab samples were stored -18°C for 12 hrs, to remove their carapaces easily. They were washed with tap water, dis-carapaces and viscera were manually removed. Crab flesh were carefully rewashed, drained with plastic net and analyzed.
Analytical methods
Chemical composition
Chemical composition of crab meat; moisture content of crab meat by a drying oven, at 105°C for overnight, total crude protein (total N×6.25) by Kjeldahl apparatus, total crude fat by Soxhlet apparatus using petroleum ether, and ash content by a muffle furnace at 550°c till obtained a constant weight were determined according (AOAC, 2005).
Quality indices
pH value
The pH values of raw crab meat were measured were estimated by pH meter (Orion pH meter Model 420 A) as the method described by Zaika et al. (1976).
Total volatile bases-nitrogen (TVB-N) content
TVB-N content was estimated by macro-distillation method of Person (1976) was determined as follows: 10g of minced crab meat was macerated with 100 ml. of distilled water. Two grams of magnesium oxide (MgO) and antifoaming substances were added and the distillation of ammonia was carried out. The ammonia received in boric acid solution (2%) containing methyl red as indicator and titrated by sulphuric acid (0.1N). The results were expressed as mg/100g sample.
TVB-N mg/100g sample = (V × N × 14 / sample wt.) ×100
Where; V: Volume taken of H2So4; N: Normality of H2So4 (0.1 N); The results obtained were expressed as mg TVB-N mg/100g sample (wet wt.).
Trimethylamine-nitrogen (TMA-N) content
TMA-N was determined calorimetrically using the standard method as described in the (AOAC, 2005). The results obtained were expressed as mg TMA-N mg/100g sample (wet wt.).
Thiobarbituric acid (TBA) value
TBA value was estimated as the method described by (Tarladgis et al., 1960) as follows: Ten grams of sample were blended with 97.5 ml of distilled water and 2.5 ml of 4N HCL and distilled and then 50 ml of distillate into test tube, sealed were obtained. After that, 5 ml of the distillate was added to 5 ml of TBA reagent (0.2883 g TBA/100 ml of 90% glacial acetic acid) into stopper tube, and then the mixture was heated in a boiling water bath for 35 minutes. After cooling at the ambient temperature, the absorbance was measured at 538 nm using digital Spectrophotometer (J.P. SELECTIA S.A) against a blank which carried out in the same manner using 5 ml distilled water with 5 ml. TBA reagent. Then, the TBA value was calculated as mg. Malonaldehyde /kg sample (wet wt.) according to the following equation:
TBA value (mg MDA\kg sample)= 7.8×Absorbance (at 538 nm)
Where; 7.8: constant.
Amino acids composition
Total amino acids (TAAs) were determined according to the methods described by (Moore et al., 1958) as follows: Sample of 20-25mg was placed in glass hydrolysis tube containing 10 ml of 6 N HCl with 0.1% mercaptoethanol. The tube was sealed and heated in an oven at 110 oC for 24 hrs. The hydrolyzed sample was then cooled to room temperature and filtered through Whatman No. 1 filter paper. The tube and precipitate on the paper was washed with distilled water and the filtrates were then completed to a 25ml in a volumetric flask. Five ml of the filtrate were transferred to a 25ml beaker and placed under vacuum in a desiccator over Potassium hydroxide (KOH). The resulted dried residue was dissolved in one ml of sodium citrate buffer of pH 2.2 and stored at 4°C until analyzed by Beckman Amino Acid Analyzer Model 119 CL. The results obtained were expressed as g/100g sample (on dry wt. basis). This determination was done at amino acids Lab., Agricultural Research Center, Cairo, Egypt.
Determination of fatty acids
The method of (AOAC, 2000) was conducted for lipid extraction from sample using chloroform methanol (2:1 v/v). The lipid samples were saponified over-night with ethanoic KOH (20%) at room temperature (Vogel, 1975). In order to have more representative samples, lipid extracts from crab samples were pooled together for preparation of fatty acid methyl esters (FAME) and two such pooled samples were analyzed. The lipids were trans methylated using ethanoic koh (20%) at room temperature to obtain FAME. FAME was analyzed by (a Pye Unicam series 304 Gas Chromatography) for identifying the individual fatty acids. The separation of fatty acid methyl esters was conducted using a coiled glass column (1.5m×4 mm) packed with Diatomite (100-20 mesh) and coated with 10 % polyethylene glycol adipate (PEGA). The column oven temperature was programmed at 8 oC/min from 70ºC to 190ºC, then isothermally at 190ºC for 25 min with nitrogen at 30 ml/min (Farag et al., 1986).
Volatile aromatic compounds
Volatile aromatic compounds were determination by Gas chromatography according to the methods described by Gu et al. (2013). Analytical of volatile compounds was performed by using a Perkin Elmer Auto system XL equipped with flame ionization detector (FID). A fused silica capillary column ZB5 (60m x 0.32 i.d.) was used. The oven temperature was maintained initially at 50°C to 240°C at a rate of 30°C/min. Helium was used as the carrier gas, at flow rate 1.1ml/min. The injector and detector temperatures were 230 and 250°C, respectively. This determination was done at Flavors, Taste and Smell Lab., National Research Center, Cairo, Egypt.
Minerals composition
Minerals composition; Na, K, Ca, ph, Fe, Mg, Mn and Zn were determined according to the methods described by Walsh (1955) using an atomic absorption spectrophotometer (ICE 3000 SERIES, England). 1g of oven-dried crab sample was put in combustion degree oven 500°C for two days. The samples were digested with 3 ml concentrated nitric acid and 1 ml of perchloric acid. The mixture was placed on a water bath till a clear colour was obtained. So the sample is ready to estimate the concentration of the elements steps measurements device. This determination was done at Central Lab., Faculty Agriculture, Zagzig University.
Statistical analysis
The results obtained were subjected to descriptive statistics and tested using analysis of variance and Duncan’s multiple range tests using SPSS version 20 Statistical Package for Windows (Differences were considered to be significant when p<0.05).
RESULTS AND DISCUSSION
Table 1 shows the proximate analysis and quality criteria of raw blue crab flesh. The chemical composition (ww) of crab meat was 83.59% moisture, 9.12% crude protein, 1.80% lipid, 1.22% ash and 4.27% carbohydrates content. Our results are lower content of crude protein, lipid and ash than 18.53%, 3.27%, 5.74%, respectively of crab (Chiromantes boulengeri) (Khaled and Khafaji, 2018) and 15.30%, 2.30%, 5.70%, respectively of crab (Atergatis roseus) (Salama and Mona, 2018). However, moisture was higher content than (73.50 – 76.40%) those findings by the same authors. Concerning carbohydrates, it is lowest content than 2.70% of estuarine crab (Scylla serrata) (Jelin and Keerthika, 2017) and also 2.10% of swimming crab (Charybdis smithii) (Yogesh et al., 2019).
Table 1: Chemical composition and quality indices (ww) of blue swimmer crab meat.
| Item | Value (%) |
| Moisture | 83.59±0.16 |
| Protein | 9.12±072 |
| Lipid | 1.80±0.76 |
| Ash | 1.22±0.81 |
| Carbohydrate | 4.27±0.05 |
| pH value | 7.35±0.06 |
|
1TVB-N (mg\100 g) |
47.42±9.69 |
|
2TMA-N (mg\100 g) |
0.19±0.01 |
|
3TBA (mg MA\kg sample) |
0.15±0.36 |
1TVB: total volatile basic nitrogen; 2TMA-N: trimethylamin nitrogen; 3TBA: thiobarbituric acid.
This variation in chemical composition of crab is affected by season and location of catch, feeding, crab species, age, sex etc. (Barrento et al., 2010). With regard to quality indices of crab meat (Table 1), values of pH, TVB-N, TMA-N and TBA of crab flesh were 7.35, 47.42 mg/100g, 0.19mg/100g and 0.15mgMDA/kg sample, respectively. The pH value was higher than 6.60 (Mehta and Nayak, 2017), 6.16 (AbouZeed, 2016) and 8.50 (Ibrahim, 2017) of some different crustaceans species. Also, the TVB-N content was higher than 4.06 and 4.86 mg/100g of crayfish and shrimp, respectively (Ibrahim, 2017). TBA content was lower than 0.63 and 0.79 mg/100g of crayfish and shrimp, respectively (Ibrahim, 2017). Also, the TBA content was lower than 0.25 MDA/kg sample (Shimaa et al., 2014). Our data showed that TVN value was high compared with previous studies, may be refer to handling steps of crab from catch to marketing.
Amino acids composition of raw blue crab meat
Amino acids composition (DW) of raw blue crab meat is presented in Table 2. It could be found that total essential amino acids (EAAs) composition of raw blue crab meat was 21.8mg\sample and included 7 AAs. The values of EAAs were threonine 2.92, valine 2.94, methionine 1.86, leucine 4.71, phenylalanine 2.69, histidine 1.55, and lysin 5.13 mg/100g.
Table 2: Essential (EAAs) and Nonessential (NEAAs) amino acids of crab meat (DW).
| EAAs | mg/100g | NEAAs | mg/100g |
| Threonine | 2.92 | Serine | 2.39 |
| Valine | 2.94 | Glutamic | 9.96 |
| Methionine | 1.86 | Glycine | 4.58 |
| Leucine | 4.71 | Aspartic | 5.75 |
| Phenylalanine | 2.69 | arginine | 6.18 |
| Histidine | 1.55 | Proline | 3.04 |
| Lysin | 5.13 | Alanine | 4.76 |
| Tyrosine | 2.50 | ||
| Cysteine | 0.98 | ||
| Total EAAs | 21.8 | Total non-EAAs | 40.14 |
EAA: Enessential amino acids; NEAA: Nonessential amino acids.
In this study, total EAAs of blue crab meat was higher content than 2.657mg\100g (Silambarasan et al., 2016) and also 5.56 mg\100g (Wilson et al., 2017). However, total content is lower than 22.34mg\100g (El-Gendy et al., 2018) and also 34.70 mg\100g (Moruf and Lawal-Are, 2019). Moreover, total nonessential amino acids (NEAAs) composition of crab meat was 40.41mg\100g sample and involved 9 AAs. The values of NEAAs were serine 2.39, glutamic 9.96, glycine 4.58, aspartic 5.75, arginine 6.18, proline 3.04, alanine 4.76, tyrosine 2.50 and cysteine 0.98 mg/100g.
Our results are higher than 3.705mg\100g for only 7 NEAAs of rainbow crab (Silambarasan et al., 2016) and 5.92mg\100g of swimming crab (Wilson et al., 2017) however, they are lower content than 19.98mg\100g of green tiger prawn (Penaeus Semisulcatus) (El-Gendy et al., 2018) and 26.15mg\100g of crab (Callinectes amnicola) (Moruf and Lawal-Are, 2019) for only glutamic, aspartic and cysteine.
Fatty acids composition of raw blue crab meat
Data in Table 3 show the saturated (SFAs) and unsaturated fatty acids (USFAs) composition (DW) of raw blue crab meat. Total SFAs of crab meat was 2733.67 mg/100g sample, included 8 FAs. Values of SFs were stearic 2354.63, arachidic 46.28, pelargonic 91.66, heneicosanoic 3.63, lignoceric 174.47 and behenic 63.00 mg\100g while both Undecylic and tridecylic were not detected. On the other side, total USFAs was 1061.93mg\100g, included also 8 FAs. Values of USFs were nondecylic 274.41, eicosenoic 8.82, nervonic 319.50, oleic 56.69, linolenic 24.60, Linoleic 196.79, eicosadienoic 179.90 and tricosanoic 1.22 mg/100g of crab meat.
Table 3: Saturated (SFAs) and unsaturated (UFAs) fatty acids of raw crab meat.
|
1SFAs |
mg\100g |
2UFAs |
mg\100g |
| Stearic (c18:0) | 2354.63 | Nondecylic (c19:0) | 274.41 |
| Arachidic (c20:0) | 46.28 | Eicosenoic(c20:2) | 8.82 |
| Pelargonic (c9:0) | 91.66 | Nervonic (c24:1) | 319.50 |
| Undecylic(c11:0) |
*ND |
Oleic(c18:1) | 56.69 |
| Tridecylic (c13:0) |
*ND |
Linolenic (c18:3) | 24.60 |
| Heneicosanoic(c21:0) | 3.63 | Linoleic(c18:2) | 196.79 |
| Lignoceric (c24:0) | 174.47 | Eicosadienoic(c20:2) | 179.90 |
| Behenic(c22:0) | 63.00 | Tricosanoicacid(C23:0) | 1.22 |
| Total SFAs | 2733.67 | Total UFAs | 1061.93 |
1SFAs: saturated fatty acids; 2UFAs: unsaturated fatty acids. *ND: not detected.
These results especially stearic and oleic acids (2354.63, 56.69 mg\100g) are higher than 8.26 and 2.17 (Silambarasan et al., 2016), 14.6 and 15.72 (Sreelakshmi et al., 2016), 5.95 and 0.98 (Mehta and Nayak, 2017) and 9.45 and 17.62mg/100g (Moruf and Lawal-Are, 2019). Similar trend is found in case of arachidic acid (46.28 mg/100g) compared with 0.03 mg\100g (Silambarasan et al., 2016) and 0.016 (Moruf and Lawal-Are, 2019). And also Linoleic (196.79mg\100g) is more content than 15.85 (Ramamoorthy et al., 2016), 1.04 mg\100g (El-Gendy et al., 2018) and 3.67 mg\100g (Yogesh et al., 2019). Similar results are found in both behenic and linolenic acids compared with those reported by Silambarasan et al., (2016) and Moruf and Lawal-Are (2019).
Volatile aromatics compounds of blue crab meat
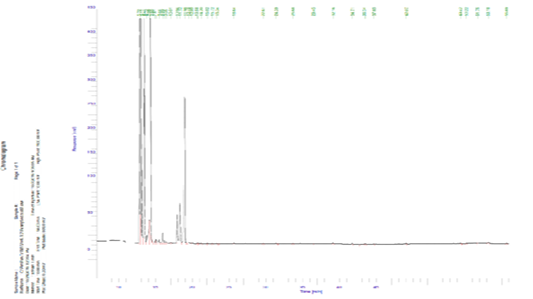
Figure 1 and Table 4 and show that 23 components of volatile aromatic compounds (ww) of raw blue crab meat were determined and identified. These compounds could be divided into 7 groups.
The values of total aldehydes, aromatics, alcohols, furans, N-containing compounds, ketones and hydrocarbon compounds were 34.91, 0.15, 0.06, 0.03, 29.76, 34.75 and 0.05, respectively. The groups compounds were taken the following order; aldehydes, ketones, N-containing compounds. These results are within with several authors; Giogios et al. (2013) and Gu et al. (2013) who found that the values of total volatile aromatic compounds ranged 0.45-1637.67 aldehydes, 0.29-140.36 aromatics and not detected-45.49 hydrocarbon, respectively of shrimp (Parapenaeus longirostris) and male Chinese mitten crab (Eriocheir sinensis).
Table 4: Volatile compounds of blue crab meat.
| Compound | Relative area (%) |
| Aldehydes | |
| Acetaldehyde | 7.97 |
| 2- Methyl butanal | 0.20 |
| 3- Methyl butanal | 0.26 |
| 2-Pentanal | 0.07 |
| Hexanal | 1.99 |
| 2- Hexanal | 24.28 |
| 2-Furfural | 0.03 |
| 4-Heptenal | 0.05 |
| Heptenal | *ND |
| Octanal | *ND |
| Nonanal | 0.03 |
| Pentadecanal | 0.03 |
| Total | 34.91 |
| Aromatics | |
| Toluene | 0.07 |
| Xylene | *ND |
| Methyl naphthalene | 0.08 |
| 4-Methylphenol | *ND |
| Total | 0.15 |
| Furans | |
| Butyl furan | 0.03 |
| Total | 0.03 |
| N-containing compounds | |
| 2,5-Dimethyl pyrazide | 0.03 |
| Trimethylamine | 29.73 |
| Total | 29.76 |
| Ketones | |
| Butandione | 34.75 |
| 1-Penten-3-one | 0.07 |
| Total | 34.82 |
| Hydrocarbon | |
| Pentadecane | 0.05 |
| Total | 0.05 |
*ND: not detected.
Minerals composition of crab meat
Table 5 shows the minerals composition (DW) of blue crab meat. The levels of minerals were 1.98, 1.23, 0.29, 0.22, 0.44 and 0.01mg\100g, respectively of Sodium (Na), Potassium (K), Calcium (Ca), Magnesium (Mg), Phosphorous (P) and Zinc (Zn). These results are lower than those reported by Silambarasan et al. (2016); Wilson et al. (2017) and Yogesh et al. (2019); they found that Ca 216.8, 218.8 and 187.9 mg\100g, Na 102.4, 45.6 and 317.1 mg\100g, K 136.9, 59.43 and 148 mg\100g, Zn 9.84, 2.33 and 1.74 mg\100g, respectively. And also, Wilson et al. (2017) and Yogesh et al. (2019); they found that Mg and P levels were 3.9 and 34.31 mg\100g of swimming crab (portunus sanguinolentus) and swimming crab (Charybdis smithii).
Table 5: Minerals of crab meat.
| Element | mg\100g |
| Sodium | 1.98 |
| Potassium | 1.23 |
| Calcium | 0.29 |
| Magnesium | 0.22 |
| Phosphorous | 0.44 |
| Zinc | 0.01 |
CONCLUSION
Based on the results obtained, total volatile basic nitrogen (TVB-N) of crab meat was a high content but it does not exceed the permissible level of crustaceans. Crab meat contained 19 volatile aromatics compounds, 7 essential amino acids (EAAs), 9 nonessential amino acids (NAAs), 8 saturated fatty acids (SFAs) and 7 unsaturated fatty acids (USFAs). Also, total SFAs (was higher than total USFAs. In addition, it contained accepted levels of Na, K, P, Ca and Mg. Generally, this study recommends that blue crab meat is an important source of valuable nutrients, AAS, FAs and major elements.
Authors Contribution
All authors contributed equally to this article.
Conflict of interest
The authors have declared no conflict of interest.
References