Advances in Animal and Veterinary Sciences
Research Article
Prevalence and Distribution of Enterotoxin Genes among Bacillus cereus Isolated from Meat and Meat Products in Egypt
Ahmed E. Tharwat1, Nesreen Z. Eleiwa2, Nada S.M. Ali1, Abdallah M.A. Merwad3*
1Department of Food Control, Meat Hygiene, Faculty of Veterinary Medicine, Zagazig University, Egypt; 2Chief Researcher of Food Hygiene, Animal Health Research Institute, Doki Cairo, Egypt; 3Department of Zoonoses, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Abstract | This study determined the incidence and distribution of two enterotoxin genes in Bacillus cereus isolates originating from meat and meat products. A total of 100 samples of meat, including minced meat, luncheon, sausage and pastrami (25 each), were randomly collected from different supermarkets at Gharbia Governorate, Egypt. The samples were cultured individually on mannitol-egg yolk-polymyxin (MYP) agar plates and sheep blood agar plates. Biochemical identification of presumptive colonies was performed. The cytotoxin K (cytK) and hemolysin BL (hblC) genes were detected in B. cereus strains (n=12) from minced meat and meat products by multiplex PCR. B. cereus was identified in 45% of the collected samples. A higher prevalence of B. cereus was detected in minced meat samples (76%) followed by luncheon (44%) and sausage (32%), while the lowest prevalence was observed in pastrami samples (28%). Pairwise comparisons showed significant differences in the distribution of B. cereus isolates between minced meat and pastrami samples (P=0.004), and minced meat and sausages(P=0.01). The co-occurrence of both cytK and hblC (66.7%) was dominant among enterotoxin genes, followed by cytK (25%) and hblC (8.3%). A higher incidence rate of B. cereus in minced meat indicates cross-contamination during processing and transportation, and therefore, represents a significant public health hazard for consumers.
Keywords | Bacillus cereus, Meat products, Cytotoxin K gene, Haemolysin BL gene, Prevalence
Received | June 10, 2020; Accepted | June 25, 2020; Published | July 10, 2020
*Correspondence | Abdallah M.A. Merwad, Department of Zoonoses, Faculty of Veterinary Medicine, Zagazig University, Egypt; Email: merwad.abdallah@yahoo.com
Citation | Tharwat AE, Eleiwa NZ, Ali NSM, Merwad AMA (2020). Prevalence and distribution of enterotoxin genes among Bacillus cereus isolated from meat and meat products in Egypt. Adv. Anim. Vet. Sci. 8(s1): 41-46.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s1.41.46
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Tharwat et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bacillus cereus is a Gram-positive, spore-forming bacterium that induces food poisoning in the forms of emetic and diarrheal syndromes (Kim et al., 2009; Arslan et al., 2014). The syndrome of diarrhea usually occurs within 8-16 hours after consumption of contaminated food (Park et al., 2009). The pathogen is widely distributed in different types of foods comprising cooked rice, vegetables, fish, meat, and milk products (Dzieciol et al., 2013). Raw meat and meat products are relevant sources of animal protein in the diet; therefore, the contamination of fresh meat and meat products with B. cereus represents a severe public health hazard. A presence of B. cereus (103-105 CFU/gm) poses a moderate risk to initiate cases of food poisoning (Rajkovic et al., 2013). Food poisoning occurs because B. cereus spores could survive the processes of cooking and pasteurization. If the food is not adequately refrigerated, and there is a lack of competitive flora, the bacterium germinates and multiply (Kramer and Gilbert, 1989). B. cereus spores are resistant to adverse environmental conditions; it could access food of animal and plant origin and, therefore, could contaminate products of milk and meat products (Granum, 1994; Larsen and Jorgensen, 1997). The bacterium is usually associated with food poisoning and diarrhea due to the release of heat-labile enterotoxins (Granum, 1994; Forghani, 2015). The enterotoxins are comprised of hemolysin BL (Hbl), non-hemolytic enterotoxin, (Nhe), cytolysin K (CytK), and enterotoxin FM (EntFM), and are responsible for diarrhea (Kim et al., 2011; Hwang and Park, 2015). Consist of two lytic proteins (L1 and L2) and the binding component B; the Hbl toxin causes hemolysis, cytotoxicity, dermo-necrosis, and vascular permeability. The toxins (CytK and EntFM) are composed of a single-component protein. Recognized the principal virulence factor in diarrhea, the cytotoxin K (cytK) is highly cytotoxic and has the potential to cause necrosis and hemolysis (Lund et al., 2000).
B. cereus has been associated with different foodborne outbreaks (Arnesen et al., 2008; Bottone, 2010; Bennett et al., 2013). Owing to a lack of effective surveillance, B. cereus-related food poisoning may be underreported, and, due to similar clinical picture, sometimes might obscure food poisoning caused by Clostridium perfringens and Staphylococcus aureus (Arnesen et al., 2008). Therefore, this study was performed to determine the prevalence, and an incidence of enterotoxin genes (cytK and hblC)-carrying B. cereus isolates from meat and meat products at Gharbia Governorate, Egypt.
MATERIALS AND METHODS
Collection of samples
The study processed a total of one hundred beef samples from meat and meat products. These included 25 grams each of minced meat, luncheon, sausage, and pastrami. The samples were randomly collected from different supermarkets at Gharbia Governorate, Egypt, and were directly transferred to the laboratory in an icebox under complete aseptic conditions.
Isolation and identification of Bacillus cereus group isolates
Each sample of meat and meat products (10 g) was homogenized and added to 90 mL of 0.1% peptone water (Oxoid, CM0009, UK). The samples were mixed and homogenized by vortex at room temperature for 3 minutes. A 10-fold dilution was prepared in 20% (v/v) glycerol-peptone water. A 50 µL aliquot from each dilution was mixed with 5 mL of Nutrient broth (Oxoid, CM0001, UK) followed by incubation at 37°C for 18 hours with continuous shaking at 150 rpm (Rahimi et al., 2013). The tubes were subjected to pasteurization at 80°C for 10 min to eliminate non-sporulating bacteria. The suspension was streaked onto agar plates of mannitol-egg yolk-polymyxin (MYP) (Oxoid, CM0929, UK), followed by incubation at 34º C for 24-48 hrs (Banyko and Vyletelova, 2009). Presenting typical pink colonies surrounded by egg yolk precipitate on MYP plates, the presumptive B. cereus group isolates were cultured on sheep blood agar plates (Oxoid, CM0271, UK) and incubated at 34º C for 24 hrs. Bacillus cereus colonies exhibited creamy to white or grey color and had a slight green tinge on the blood agar. Those colonies that revealed beta hemolysis were further identified by biochemical tests, including catalase production, Voges-Proskauer, hydrolysis of gelatin, nitrate reduction, starch hydrolysis and glucose acidification as described previously (Tallent et al., 2012). With a final concentration of 25% in glycerol, the bacterial cultures were stored at -80°C.
PCR for analysis of enterotoxin genes in bacillus cereus group isolates
DNA extraction
B. cereus cultures were grown onto tryptic soya agar at 30ºC for 24 hrs. Cells were subjected to washing from the surface by saline solution (0.85 %). After that, separation of cells was done from the resulting suspension (1 mL) by centrifugation (5000xg for 5 min). Washing of each pellet was carried out by the addition of sterile distilled water (1 mL) and centrifugation (5000xg for 5 min). Total DNA was isolated from the resulting pellet using Genomic DNA Mini Kit (A and A Biotechnology, Poland) according to the instructions of the manufacturer and stored at -20ºC until further use.
Multiplex PCR assay
The sequences of oligonucleotide primers used for the identification of enterotoxin genes (cytK and hblC) among isolates of B. cereus are listed in Table 1 (Ngamwongsatit et al., 2008). Multiplex PCR was carried out using a final volume of 20 µL that had a template DNA (5 µL, 20 ng DNA/ µL), 1X PCR buffer [Tris-HCl (10 mM) pH 8.3 and KCl (50 m M], MgCl2 (1.5 mM), dNTP (200 µM, each), primers (0.2-0.4 µ M). PCR reactions were conducted in a Thermal Cycler (BioRad, USA) as per cycling conditions described before (Ngamwongsatit et al., 2008). The program included an initial denaturation at 95°C for 5 min, followed by 30 cycles of each of denaturation (95°C /45 sec), annealing (54°C /1 min), and an extension (72°C / 2 min). The final extension was made at 72°C / 5 min. The amplicons were separated on agarose gels (1.5%), and the estimation of amplicon size was performed using a 100 bp DNA ladder (New England Labs). B. cereus isolates originating from milk (a positive control), and Escherichia coli isolates (a negative control) were kindly provided by Zoonoses Department, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Data analysis
Differences in B. cereus prevalence among minced meat and different meat products were investigated using the Kruskal-Wallis test. Pairwise comparisons with Bonferroni adjusted P-value was used to differentiate between each pair of meat products after significant Kruskal results. A statistical significance was considered at P-value < 0.05. All data analysis was done by SPSS version 24.0 (IBM. Corp., Armonk, NY).
Table 1: Primers used for multiplex PCR detection of enterotoxin genes in Bacillus cereus group isolates from meat and meat products.
| Reference | PCR products (bp) | Sequence (5′ → 3′) | Oligonucleotide primers | Target genes |
| 565 | CGACGTCACAAGTTGTAACA | CytK(F) |
cytK |
|
| CGTGTGTAAATACCCCAGTT | CytK(R) | |||
| 695 | CCTATCAATACTCTCGCAA | HblC(F) |
hblC
|
|
| TTTCCTTTGTTATACGCTGC | HblC (R) |
RESULTS AND DISCUSSION
Bacillus cereus has been implicated in many food poisoning outbreaks characterized by emetic and diarrheal syndromes (Drobniewski, 1993); however, a few of the cases are recorded simply because the clinical symptoms are almost similar to poisoning caused by Clostridium perfringens and Staphylococcus aureus (Arnesen et al., 2008; Bottone, 2010; Bennett et al., 2013). The bacterium is widely distributed in nature and has an ability to proliferate in beef luncheon, raw meat, raw milk, and Karish cheese (Abdou et al., 2012). It could survive in meat and milk products because of its capability to form spores (Christiansson et al., 1999). In the present study, using cultural and biochemical identification procedures, B. cereus group isolates were recovered from minced meat and meat products with an overall prevalence of 45% (Table 2). Nearly similar rates were reported previously from meat products in other countries. For instance, it was found to be 42% in Bareilly, India (Agarwal et al., 1997), and 48% in Netherland (Giffel et al., 1996). However, there are studies that reported a much higher prevalence than observed in the current study. For instance, chicken and meat products showed a higher incidence rate (80%) for B. cereus in India (Kamat et al., 1989). Another study carried out in Ludhiana reported a higher incidence rate (56.3%) for B. cereus in meat products (Bedi et al., 2004). Similarly, a high prevalence of B. cereus (62.5%) was recorded in ready-to-eat food products in Taiwan (Fang et al., 2003). Contrary to this, other studies reported a lower incidence rate of B. cereus in meat products. For example, it was found to be 23.5%, 30.85%, and 36.7% in India (Willayat et al., 2007; Das et al., 2009; Tewari et al., 2015), and 26% and 38.3% in Egypt (Abd El-Tawab et al., 2015; Shawish and Tarabees, 2017). Compared to our study, such variation in B. cereus isolation with different incidence rates in meat products could be attributed to the differences in the level of hygienic practices followed in meat shops and restaurants (Tewari et al., 2015). A higher rate of recovery of B. cereus from meat products in this study could be ascribed to the ambient temperature of food storage or inadequate cooking of food before its consumption which could favor endospore germination leading to a rapid increase in B. cereus (Gilbert et al., 1974; Bryan et al., 1981).
From the results recorded in Table 2, a higher prevalence rate (76%, 19/25) of B. cereus was detected in minced meat followed by luncheon (44%, 11/25) and sausage (32%, 8/25), while a lowest recovery rate (28%, 7/25) was found in pastrami. These observations are almost in congruence with those reported previously in Egypt. For example, a prevalence rate of 72% was reported in minced meat (Abu-Elnaga, 2003), while it was found to be 30% in sausage (Eid-Amal et al., 2008), and 35% in luncheon (Ghanyem-hanan et al., 2014). The Kruskal-Wallis test showed a significant difference in the prevalence of B. cereus among four of the examined meat products [H (3)=14.2, P-value= 0.003]. The Pairwise comparisons with adjusted P-value revealed significant difference in prevalence rates of B. cereus for minced meat (mean rank=66.0) than pastrami (mean rank=42.0) (P=0.004), and minced meat (mean rank=66.0) than sausage (mean rank=42.0) (P=0.01). However, no significant differences (P >0.05) were observed for the prevalence of B. cereus in pastrami versus sausage, pastrami versus luncheon, sausage versus luncheon and luncheon versus minced meat. Similar finding has been reported previously with significantly a higher prevalence rates of 65% and 35% in minced meat and luncheon, respectively in Egypt (Ibrahim-Hemmat et al., 2014). A higher prevalence rate of B. cereus in minced meat in the present study might be attributed to storage at room temperature, high content of curing salts and spices, and cross-contamination during preparation and production (Torky-Amal, 1995). In contrast to the prevalence rates of B. cereus in our study, previous studies reported isolation of B. cereus with different infection rates from meat and meat products in many countries. In Egypt, for example, B. cereus was detected in 20%, 36.37%, 40% and 56.67% in luncheon, beef burger, sausage and minced meat, respectively (Abd-el-Tawab et al., 2015). A higher rate of isolation of B. cereus was reported from raw meat (56%), followed by ground meat (40%), soudjouck (16%) and pastrami (4%) in Turkey (Guven et al., 2006). However, another study in Libya revealed a higher incidence rate of B. cereus in beef kabab (80%), chicken kabab (60%), chicken burger (30%) and beef burger (25%) (Naas et al., 2019). A lower isolation rates of B. cereus and were detected in raw meat (27.8%) and meat products (35%) in India (Tewari et al., 2015). Our findings were contrary to a recent report from Egypt (Shawish and Tarabees, 2017), where a lower prevalence rates of B. cereus were reported in beef luncheon (15%) and beef kofta (37.5%). In the present study, B. cereus prevalence was higher in minced meat and luncheon perhaps due to the cross-contamination during meat production and preparations, the type of preparation of the products, some defects in the hygienic measures, or the type of additives or spices used (Floriştean et al., 2007). Moreover, our study highlighted that a significantly higher incidence rate of B. cereus in minced meat could be attributed to the cross-contamination during processing, transportation and marketing or the additives which are considered potential risk factors that facilitate in increasing the number of Bacillus spores, and subsequently an increased chance of an occurrence of food poisoning (Shawish and Tarabees, 2017). Thereby, considerations should be taken during raw meat processing and additives from trustful sources must be utilized.
Table 2: Prevalence of Bacillus cereus isolates from meat and meat products.
| No. of positive samples (%) | Source of samples (No.) |
| 7 (28) | Pastrami (25) |
| 8 (32) | Sausage (25) |
| 11(44) | Luncheon (25) |
| 19 (76) | Minced meat (25) |
| 45 (45) | Total (100) |
The Pairwise comparisons of each pair of meat products with adjusted P-value by the Bonferroni correction were listed as below:
Pastrami vs. sausage (P=1.00), Pastrami vs. luncheon (P=1.00). Pastrami vs. minced meat (P=0.004*), sausage vs. luncheon (P=1.00). Sausage vs minced meat (P=0.01*), luncheon vs. minced meat (P=0.142). *: When the adjusted P- value < 0.05, there were significant differences in the prevalence of B. cereus among each pair of meat products.
Table 3: Distribution of enterotoxin genes in Bacillus cereus isolates recovered from meat and meat products.
| Enterotoxin genes | No. of investigated B. cereus isolates | Source of samples | ||
|
cytK and hblC No. (%) |
hblC No. (%) |
cytK No. (%) |
||
| 3 (100) | 0 (0.00) | 0 (0.00) | 3 | Minced meat |
| 1(33.3) | 1 (33.3) | 1 (33.3) | 3 | Pastrami |
| 2(66.7) | 0 (0.00) | 1(33.3) | 3 | Luncheon |
| 2 (66.7) | 0 (0.00) | 1 (33.3) | 3 | Sausage |
| 8 (66.7) | 1 (8.3) | 3 (25) | 12 | Total |
cytK: cytotoxin K gene; hblC: haemolysin BL gene.
Pathogenicity of B. cereus is induced by various toxins. The diarrhea is correlated with an occurrence of different enterotoxins such as Hbl, Nhe, CytK and EntFM (Fagerlund et al., 2004; Ehling-Schulz et al., 2005). In this regard, multiplex PCR is a fast and reliable technique used for the confirmation of enterotoxigenic B. cereus isolates from food (Ombui et al., 2008). In the present study, twelve B. cereus isolates, three isolates from each source of meat products, were randomly selected for identification of two enterotoxin genes (cyt K and hulk). Both enterotoxin genes of B. cereus were amplified with PCR products of 565 and 695 bp, respectively (Figure 1). The overall distribution of enterotoxin genes including cytK, hblC and both cytK and hblC were found to be 25% (3/12), 8.3% (1/12) and 66.7% (8/12), respectively (Table 3). Contrary to this, a higher distribution of cytK gene were reported previously from meat and food products. For example, it was found to ve 50% in Netherland (Wijnands et al., 2006), 65.9% in India (Rather et al., 2012), 1.4% in India (Tewari et al., 2015), 71% in China (Li et al., 2016) and 100% in Egypt (Shawish and Tarabees, 2017). Compared to an overall distribution of hblC gene among B. cereus isolates in our study (8.3%), a rate of incidence (90%) of hblC was reported in a recent research from Egypt (Shawish and Tarabees, 2017). The present study also showed that all three isolates (100%) of minced meat contained both cytk and hblC genes. Moreover, luncheon and sausage had similar distribution rates for cytk gene (33.3% each), and for both cytK and hblC genes (66.7% each). Pastrami samples had an equal distribution rate (33.3%) for each of cytK, hblC and both cytk and hblC genes (Table 3). This study confirmed that B. cereus isolates derived from minced meat and meat products were virulent, toxigenic and contained diarrheal genes (cytK and hblC).
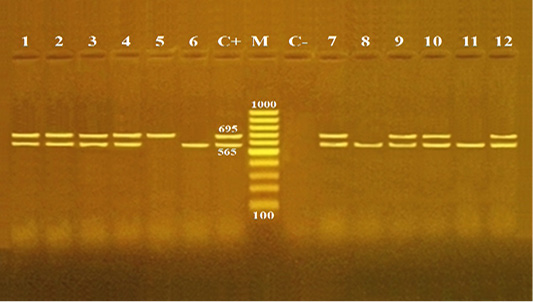
Figure 1: Agarose gel electrophoresis for multiplex PCR products of enterotoxin genes (cytK and hblC) among Bacillus cereus isolates recovered from meat and meat products.
Lane M: 100 bp DNA Ladder; lane C+: positive control B. cereus isolate for both cytK and hblC genes; lane C-: negative control Escherichia coli isolate; lanes 1-3: B. cereus isolates from minced meat; lanes 4- 6: B. cereus isolates from pastrami; lanes 7, - 9: B. cereus isolates from luncheon; lane and lanes 10, - 12: B. cereus isolates from sausage .The PCR amplicon sizes are indicated beside the bands; 565 and 695 bp for cytK and hblC genes, respectively.
CONCLUSION
This study confirmed a higher prevalence rate of B. cereus group isolates in minced meat suggesting potential cross-contamination during processing and transportation or the contamination of additives. B. cereus isolates were virulent, toxigenic and contained two virulence genes (cytK and hblC) responsible for diarrheal syndrome. Multiplex PCR proved itself a rapid technique for detection of toxigenic B. cereus isolates originating from meat products. Therefore, hygienic measures need to be strictly ascertained, and more attention should be taken during raw meat processing with the inclusion of additives exclusively from trustful sources.
Authors Contribution
Authors had participated equally in the practical work, manuscript writing and revision and statistical analysis of data.
Conflict of Interest
All authors did not have any conflict of interest to declare.
REFERENCES





