Advances in Animal and Veterinary Sciences
Research Article
Immunological Stimulant Effect of Linseed Oil and Fennel Oil Supplemented Diet on Broilers
Sherif M. Shawky1*, Said I. Fathalla1, Ibrahim S. Zahran2, Khalid M. Gaafar3, Mohamed K. Hussein4, Ibrahim S. Abu-Alya1
1Department of Physiology, Faculty of Veterinary Medicine, University of Sadat City, Egypt; 2Department of Physiology, Faculty of Veterinary Medicine, Aswan University, Egypt; 3Department of clinical nutrition, Faculty of Veterinary Medicine, University of Sadat City, Egypt. 4Department of food control, Faculty of Veterinary Medicine, Aswan University, Egypt.
Abstract | This study was designed to assess the effect of Linseed oil and Fennel oil supplemented diet on the immunity of Cobb broiler chickens through one day age to 35 days. A total of 90 broiler chicks were allocated to 3 treatment groups with 3 replicates of ten birds each. The control group (G1) was fed a basal diet. The Linseed oil group (G2) was fed the basal diet supplemented with 0.2 ml of linseed oil/Kg ration. The Fennel oil group (G3) was fed the basal diet supplemented with 0.3 ml of fennel oil/Kg ration. Feeding on diet supplemented with linseed oil and fennel oil significantly increased WBCs, Heterophils %, phagocytic activity %, phagocytic index, total protein, globulins, IgM, IgG, A/G ratio, T3, and T4 of broiler chicks compared to control group. There was a non-significant difference in Ca, Ph, albumin, AST, ALT, urea, and creatinine levels between all groups. The histological examination of the duodenum in linseed oil treated group (G2) and Fennel oil treated group (G3) compared to the control group showing normal intestinal villi with an increase in goblet cells count. In conclusion, the daily supplementation of Linseed oil and Fennel oil in the diet of broiler chicks for 35 days was enough to improve their immunity and subsequent biochemical parameters.
Keywords | Broiler, Immunity, Fennel oil, Linseed oil, Thyroid hormones
Received | May 19, 2020; Accepted | June 21, 2020; Published | June 25, 2020
*Correspondence | Sherif Mohamed Shawky, Department of Physiology, Faculty of Veterinary Medicine, University of Sadat City, Egypt; Email: shsh00076@yahoo.com
Citation | Shawky SM, Fathalla SI, Zahran IS, Gaafar KM, Hussein MK, Abu-Alya IS (2020). Immunological stimulant effect of linseed oil and fennel oil supplemented diet on broilers. Adv. Anim. Vet. Sci. 8(7): 771-776.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.7.771.776
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Shawky et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Poultry is one of the most productive and fast-growing industries. Different strategies have been applied to reduce disease outbreaks and improve immunity (Visek, 1978). In the poultry industry, herbs and herbal products are used to substitute synthetic products to stimulate or promote the effective use of feed nutrients which may result in faster body weight gain, higher rates of production, and improved feed quality. Additionally, the active ingredient of herbs may improve digestion and promote the immunity in broilers (Ghazalah and Ali, 2008).
Essential oils (EOs) have hypolipidemic, antibacterial, antioxidant, anti-inflammatory, anticarcinogenic activities (Viuda-Martos et al., 2010). Therefore, EOs is used in animal production as growth promoters (Cross et al., 2007). The EOs are usually obtained from the plant and are named according to the aromatic characteristics of the plant origin (Oyen and Dung, 1999). The dietary oil supplement is a very important supplement of polyunsaturated fatty acids (PUFA) and α-linolenic, as in linseed oil (Zelenka et al., 2006).
The most important reason for supplementation of linseed oil in the diet of broiler chickens is their favorable effect of PUFA on the health of animals and human. The primary effect of adding linseed oil is due to its high content of α-linoleic acid (Zelenka et al., 2006) and also increase in other n3 PUFA (Zelenka et al., 2008). These fatty acids are necessary for normal human growth (Simopoulos, 2001).
Fennel is a family member of the Apiaceae, which is widely cultivated in the Mediterranean region. It is an important natural, non-synthetic compound regarded as a major source of flavoring products (Yaylayan, 1991). A recent study has concluded the importance of fennel oil as a potent antioxidant, hepatoprotective, and antimicrobial agent (Ozbek et al., 2003). The supplementation of 10 or 20 g of fennel /kg diet of heat stressed laying hens significant improved the egg quality, reduced the number of broken eggs and significant decrease the malondialdehyde (MDA) levels in eggs (Gharaghani et al., 2015).
The aim of the current work is designed to assess the effect of linseed oil and fennel oil supplementation on the immunity, plasma electrolyte, liver and kidney functions, thyroid hormone levels, and histological examination of the duodenum in broilers.
MATERIALS AND METHODS
Experimental design
The experimental protocol was approved by the Animal care committee of the University of Sadat City. Ninety chicks (Cobb – strain) (90), one day old with average weight 45.0 ±2.0g obtained from El-Arabia Company for Poultry. Feed and water were provided ad-libitum, the environmental temperature was kept at 35°C in the first week, then gradually reduced by 2.3°C per week until it reached around 22°C, while the humidity was maintained at 60 %. In the poultry house, the Chicks were reared, provided with feeders, drinkers, and wood shaving which was used as bedding material. Strict sanitation practices were applied throughout the experiment. Vaccination programs were implemented according to the age of chickens. The chickens were fed starter ration from one day until the 15th day and finisher ration from the beginning of 3rd week to the end of the experiment (Table 1).
Feed additives
Linseed 0.2 ml /kg ration and fennel 0.3ml /kg ration essential oil were dissolved in 100 ml ethanol. The oil alcoholic mixtures sprays were mixed with ration then wait till complete ethanol evaporation before offering to the broiler (Rahimi and Ardekani, 2013).
Chemicals
In this experiment all the chemicals obtained from Biodiagnostic Company, Cairo, Egypt, unless otherwise mentioned. Linseed oil and Fennel oil obtained from El- Hawag Factory, Badr City, Egypt.
Table 1: Ingredients and chemical composition of the diet.
| Ingredients and composition (%) | Starter | Finisher |
| Corn | 55.59 | 61.07 |
| Soy bean meal | 37.32 | 31.83 |
| Soy oil | 2.98 | 3.41 |
| Lime stone | 1.21 | 1.42 |
| Dicalcium Phosphate | 1.60 | 1.16 |
| DL. Methionine | 0.20 | 0.10 |
| * Vitamin and Minerals | 0.60 | 0.60 |
| Sodium chloride | 0.23 | 0.18 |
| Sodium bicarbonate | 0.27 | 0.23 |
| Chemical Analysis (%) | ||
| Metabolizable energy (ME) kcal/kg | 2950 | 3050 |
| Crude Protein (%) | 21.20 | 19.16 |
| Lysine (%) | 1.14 | 1.01 |
| Methionine (%) | 0.50 | 0.39 |
| Methionine and Cysteine (%) | 1.03 | 0.84 |
| Available Methionine + Cysteine (%) | 0.85 | 0.71 |
| Calcium (%) | 0.93 | 0.90 |
| Available Phosphate (%) | 0.44 | 0.35 |
*Supplied per kilogram of diet: vitamin A, 1,500 IU; cholecalciferol, 200 IU; vitamin E, 10 IU; riboflavin, 3.5 mg; pantothenic acid, 10 mg; niacin, 30 mg; cobalamin, 10 μg; choline chloride, 1,000 mg; biotin, 0.15 mg; folic acid, 0.5 mg; thiamine 1.5 mg; pyridoxine 3.0 mg; iron, 80 mg; zinc, 40 mg; manganese, 60 mg; iodine, 0.18 mg; copper, 8 mg; selenium, 0.15 mg.
Animal grouping
The birds were randomly assigned into 3 treatment groups with 3 replicates of ten birds each. The control group (G1) was fed a basal diet. The Linseed oil group (G2) was fed the basal diet supplemented with 0.2 ml of linseed oil/Kg ration. The Fennel oil group (G3) was fed the basal diet supplemented with 0.3 ml of fennel oil/Kg ration.
Sampling
At the end of the experiment, samples of blood were collected from the wing vein into two tubes, one with anticoagulant (heparin) to determine hematological parameters including WBC’s, heterophils, phagocytic activity and index. The second tube without anticoagulant to obtain serum. Duodenum tissue samples were removed and rapidly fixed at 10% neutral buffered formalin.
Assessment of hematological parameters and phagocytic activity
All hematological parameters were done according to Feldman et al. (2000). Phagocytic activity % and phagocytic index of heterophils were done according to the method described by Sornplang et al. (2015).
Phagocytic activity % = (No of heterophils ingesting Candida X 100) / Total number of heterophils.
Phagocytic index = Total no of ingested Candida / Number of active heterophils.
Biochemical analysis
Plasma total proteins (g/l) and albumin (g/l) were carried out by a colorimetric method using commercial kits of the Diamond diagnostics following method described by Cannon et al. (1974) and Doumas et al. (1971) respectively. Protein electrophoretic fractionation profile was carried out by a Polyacrylamide Gel Electrophoresis according to Lewis et al. (2006), using commercial kits produced by Helena laboratories Serum calcium levels (Ca) were estimated using colorimeter kits according to Barnett (1965). Serum concentrations of inorganic phosphorus were estimated by colorimetric kits according to Daly and Ertingshausen (1972). Serum alanine aminotransferase (ALT) and serum aspartate aminotransferase (AST) activities were determined colorimetrically according to the method described by Reitman and Frankel (1957). Serum creatinine levels were determined by a colorimetric kit according to Young (2001). Blood urea was estimated by colorimetric kits according to Tietz (1990). Serum concentrations of thyroxin (µg/dl) and triiodothyronine (ng/dl) were measured by radioimmunoassay (RIA) as described by Robbins (1973).
Histopathological examination
After fixing of duodenum tissue samples in 10% neutral buffered formalin, they were dehydrated in ascending grades of ethyl alcohol and cleared in xylene. Specimens were embedded in paraffin wax and then sectioned by leica microtome into sections of 4 mm thickness. The prepared tissue sections were deparaffinized, stained with hematoxylin and eosin stain (Bancroft and Gamble, 2002).
Statistical analysis
All results expressed as mean ± standard error (SE). Statistical analysis was carried out by using one–way analysis of variance (ANOVA). P value of <0.05 was considered significant. The statistical analysis was done by using SPSS.
RESULTS
As shown in Table 2 the supplemented diet with linseed oil and fennel oil caused a significant increase in total WBCs count, phagocytic activity, phagocytic index, compared to the control group.
The data obtained in Table 2 also revealed that the supplementation with linseed oil has not effect on serum total protein, albumin and globulins (p<0.05) while caused a significant increase in IgM and IgG (p<0.05) compared to the control group. On the other hand, fennel oil supplementation caused a significant increase in total protein, globulin, IgM, and IgG (p<0.05) compared to the control group but has no effect on albumin.
Table 2: Immunological parameters in broilers of different treated groups.
| G1 (control) | G2 (Linseed oil) | G3 (Fennel oil) | |
|
WBCs count(103/mm3) |
13.72±0.99b |
18.80±1.01a |
19.10±0.55a |
| Heterophils % |
22.45±0.38c |
27.03±0.66b |
31.38±1.33a |
| Phagocytic activity % |
52.68±1.01b |
62.44±1.13a |
61.58±1.19a |
| Phagocytic index |
2.02±0.09b |
2.60±0.08a |
2.53±0.07a |
| Total protein (g/l) |
55.0±1.3b |
59.9±0.6ab |
69.4±1.5a |
| Albumin (g/l) |
25.5±1.0a |
25.5±0.3a |
28.5±1.1a |
| Globulin (g/l) |
29.5±0.4b |
34.4±0.4ab |
40.9±1.0a |
| IgM (g/l) |
4.87±0.03b |
8.17±0.20a |
7.63±0.37a |
| IgG (g/l) |
2.40±0.10b |
3.97±0.33a |
4.10±0.40a |
In the same row, Mean ± SE with different letters superscripts are significantly different at (P < 0.05).
The data obtained in Table 3 and 4 revealed that there is a non-significant difference (p<0.05) between treated groups and control group in AST, ALT, urea, creatinine, calcium and phosphorus while supplementation with linseed oil and fennel oil caused a significant increase in T3 and T4 compared to the control group.
Table 3: Liver and kidney function tests in broilers of different treated groups.
| G1(control) | G2 (Linseed oil) | G3 (Fennel oil) | |
| AST (U/L) |
161.67 ± 3.80a |
170.00 ± 3.65a |
1.58.33 ± 2.79a |
| ALT (U/L) |
92.67 ± 0.76a |
91.33 ± 0.84a |
92.33 ± 0.92a |
| Urea (mg/dl) |
1.68 ± 0.02a |
1.73 ± 0.02a |
1.75 ± 0.04a |
| Creatinine (mg/dl) |
1.81 ± 0.04a |
1.79 ± 0.03a |
1.78 ± 0.03a |
In the same row, Mean ± SE with different letters superscripts are significantly different at (P < 0.05).
Table 4: Thyroid hormones and electrolytes in broilers of different treated groups.
| Groups | G1(control) | G2 (Linseed oil) | G3 (Fennel oil) |
| T3 (ng/dl) |
33.97 ± 0.97c |
43.47 ± 0.96b |
50.67 ± 1.43a |
|
T4 (µg/dl) |
2.9 ± 0.11b |
4.91 ± 0.17a |
5.11 ± 0.08a |
| Calcium (mg/dl) |
10.82 ± 0.08a |
10.56 ± 0.13a |
10.18 ± 0.30a |
| Phosphorus (mg/dl) |
2.60 ± 0.09a |
2.20 ± 0.12a |
2.34 ± 0.11a |
In the same row, Mean ± SE with different letters superscripts are significantly different at (P < 0.05).
Duodenum histopathology
The histological examination of duodenum revealed that in linseed oil group (G2) and fennel oil group (G3) there are normal intestinal villi with an increase in goblet cell count.
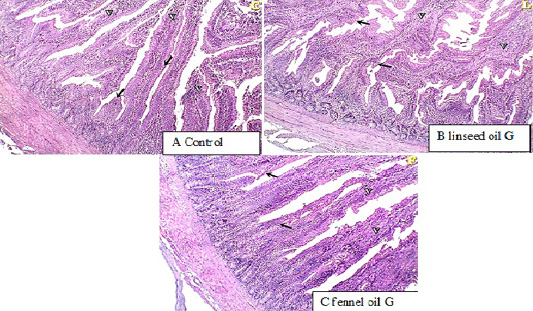
Figure 1: histopathological examination of duodenum showed normal intestinal villi with an increase in goblet cell count in linseed oil group (G2) and fennel oil group (G3).
DISCUSSION
The results of the present study showed that the supplemented diet with linseed oil in the diet caused a significant increase in total WBCs count, phagocytic activity, phagocytic index, IgM IgG, T3, and T4 while there is a non-significant difference (p<0.05) in total protein, albumin, globulin, AST, ALT, urea, creatinine, calcium, and phosphorus in compared with the control group. On the other hand, supplementation with fennel oil in G3 caused a significant increase in total WBCs count, phagocytic activity, phagocytic index, total protein, globulins, IgM IgG, T3 and T4 while there is a non-significant difference (p<0.05) in albumin, AST, ALT, urea, creatinine, calcium, and phosphorus in compared with the control group. the present results which revealed the hematological effects of linseed oil and fennel oil supplemented diet on broilers are in agree with Ragab et al. (2013) and Shunthwa et al. (2017) who reported that the use of linseed oils at different levels as a replacement to sunflower oil in the diet of broiler chickens significantly increased WBCs count, heterophils % and eosinophils %. This may be attributed to its direct effect on the hemopoietic organ (Khodary et al., 1997). Ragab et al. (2013) stated that dietary supplementation of 1 or 2% of fennel seeds improved significantly leukocytes, and meat breast (%) under elevated temperature in Ross broilers. Additionally, Gharaghani et al. (2015) noticed the potential effect of fennel fruits as an anti-stressor agent in heat-stressed laying hens. The results of the Linseed oil their impacts on the immunity, total protein and globulins were agree with Corduk et al. (2013) who showed that the supplementation of phytoadditives had no significant effect on the serum total protein. While, effect of fennel oil supplementation agreed with other poultry studies, the rise in the plasma total protein was found as a result of the addition of phytoadditives (oil of oregano or red pepper) (Elagib et al., 2012; Amad et al., 2013). Furthermore, Abdel-fattah et al. (2008), Nasir and Grashorn (2010) found that the increase in the globulin fraction indicates the effective role of using supplementation substances in enhancement the immunity due to its role in developing and protecting cells and inhibiting non-enzymatic oxidation.
Zeng et al. (2014) reported that the diet supplemented with essential oil could improve the immune status by increasing phagocytosis, lymphocyte proliferation rate, IgG, IgA, and IgM serum levels. The increased levels of immune globulins (IgM or IgG) in piglets fed diet supplemented with linseed oil may enhance immune function and assist in development of their immune system, thereby alleviating the stress. The explanation of this might be due to presence of PUFA in the oil supplemented diet that could help in modulation of immune stress. Moreover, the mixture of plant oil (MPO) may also influence the local immune response by controlling the gut microbial ecosystem, which remained to be further investigated. (Long et al., 2020). The dietary supplemented with plant mixture oil containing PUFA like linseed oil can help improved plasma immunoglobulin which may enhance the immune response in animals (Abdulla et al., 2019).
As well as, the administration of feed additive (Marjoram) caused a significant increase (P˂0.05) in gamma globulin and act as an immunity stimulator for broiler chicks (Shawky et al., 2020). The present study showed that the supplementation of linseed oil and fennel oil in the diet of broilers lead to a significant increase in serum T3 and T4 levels. Thyroid hormones play a significant role in regulation of bird metabolic rates during growth and egg production (May et al., 1986). Serum T3 and T4 elevation as an indicator for thyroid gland activity during the stage of the immune response and T3 appears to be the primary thyroid hormone involved in immunoregulation. The increase in thyroid hormones may be necessary to provide the extra energy needed for the differentiation of B cells to plasma cells and the resulting production of antibodies (Trout et al., 1988).
The present results are in a line with Golian and Kermanshahi (2015) who reported that the addition of herbal extract significantly increased plasma T3 concentration compared to those fed control diet. Also, Sahin et al. (2003) found a significant increase in T3 levels in chickens fed vitamin C and chromium under high temperature compared to their control. This may explain that the antioxidant supplementation may be favorable to counteract the negative effects of heat stress on the thyrotrophic axis.
Histopathological examination of duodenum showing normal intestinal villi with an increase in goblet cells count in Linseed oil treated group (G2) and fennel oil group (G3). The increase in the goblet cell number may be also as an immune response against the anti-nutrients (Marchetti et al., 2006). The supplementation with plant mixture oil containing linseed oil has positive effects on antioxidant capacity due to functional fatty acid, particularly n-3 PUFA in linseed oil of MPO, which can alleviate cell oxidation and improve intestinal health (Kim et al., 2007). The concentrations of PUFA may impair oxidative stress in the intestinal villi which are the site for nutrient absorption. The improvement in the intestinal morphology may be the reason that supplementation with plant extract oil like linseed can decrease pathogenic bacteria in the gut, which may help enhance the proliferation of epithelial cells to build villus (Mourao et al., 2006). Another explanation of the present study may be due to that fennel oil and linseed oil can generate rapidly available energy for duodenal villi and intestinal tissues in broilers.
This result matched with Hlophe and Moyo (2014) who observed that a significant increase in goblet cell number in fish fed Moringa herbal extract.
CONCLUSION
This study indicated the beneficial use of linseed oil and fennel oil in the diet of broiler. They could improve the immunological status and immunological parameters in broiler chickens in addition to improving thyroid hormones.
Acknowledgements
We would like to thank the Faculty of Veterinary Medicine, University of Sadat City for their support.
Authors Contribution
Sherif M. Shawky; Said I. Fathalla and Khalid M. Gaafar designed the study, wrote the protocol, and wrote the first draft of the manuscript. Sherif M. Shawky; Ibrahim S. Abu-Alya; Mohamed K. Hussein, and Ibrahim S. Zahran managed the analyses of the study. Sherif M. Shawky and Ibrahim S. Abu-Alya managed the practical work, performed the statistical analysis and the literature searches. All authors read and approved the final manuscript.
Conflict of interest
The authors have declared no conflicts of interest.
REFERENCES





