Advances in Animal and Veterinary Sciences
Research Article
Extraction of Tannin from Acacia (Acacia mangium) Bark and its use as a Feed Additive for Protecting in vitro Ruminal Degradation of Tofu Dregs
Tekad U.P. Sujarnoko1,3, Roni Ridwan2, Nahrowi1, Anuraga Jayanegara1*
1Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia; 2Biotechnology Research Center, Indonesian Academy of Sciences, Jl. Raya Bogor Km 46, Cibinong, Bogor 16911, Indonesia; 3Graduate School of Nutrition and Feed Science, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia.
Abstract | This experiment aimed to extract tannin from acacia bark by using a hot water extraction system at different temperature and pressure. Further, the tannin extract was used as an additive for protecting tofu dregs from microbial degradation in an in vitro rumen fermentation system. Acacia bark was extracted for its tannin by using a hot water extraction system, conducted at different temperature (70 and 120oC) and pressure (1 and 2 bar) for 2 h period. Samples were determined for their phenol, tannin, condensed tannin (CT) and hydrolysable tannin (HT). Tannin extract was added to tofu dregs at various levels, i.e., tofu dregs without tannin (T0), tofu dregs + 1% acacia tannin (T1), tofu dregs + 2% acacia tannin (T2), tofu dregs + 3% acacia tannin (T3), and tofu dregs + 4% acacia tannin (T4). These treatments were subjected to in vitro incubation with buffered-rumen fluid for 48 h. Results revealed that phenol, tannin, CT and HT contents in acacia bark were higher (P<0.001) when extracted at 120oC as compared to that at 70oC. The addition of tannin at 3 and 4% reduced the gas production of tofu dregs right from the early incubation period to the end in comparison to that of control treatment (P<0.05). Total VFA and ammonia concentrations were reduced by 19.9 and 58.6% (P<0.05), respectively, in the addition of 1% tannin extract. Higher levels of tannin additions led to further decrease of these parameters. Similarly, IVDMD and IVOMD of tofu dregs were reduced due to acacia tannin extract addition at 1 to 4% (P<0.05). In conclusion, tannin extract from acacia bark is able to protect microbial degradation of tofu dregs in the rumen environment.
Keywords | Tannin, Extraction, Acacia, Fermentation, Rumen
Received | April 21, 2020; Accepted | June 21, 2020; Published | June 25, 2020
*Correspondence | Anuraga Jayanegara, Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia; Email: anuraga.jayanegara@gmail.com
Citation | Sujarnoko TUP, Ridwan R, Nahrowi, Jayanegara A (2020). Extraction of tannin from acacia (Acacia mangium) bark and its use as a feed additive for protecting in vitro ruminal degradation of tofu dregs. Adv. Anim. Vet. Sci. 8(7): 761-765.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.7.761.765
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sujarnoko et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Metabolic process in the rumen occurs in anaerobic condition with the help of microbes (Broucek, 2014). This mechanism is efficient to producing quality food from organic materials that are difficult to digest. The presence of microbes in the rumen provides microbial protein as a main nutrient source, particularly protein, for further utilization by animals. On the other hand, rumen microbes may decrease quality of protein by degrading it into various amino acids and subsequently converting the amino acids into α-keto acid and ammonia in a process so called deamination (Ben Salem et al., 2005). Deamination reduces the quality of protein and therefore the process should be reduced or decelerated especially for high quality feed ingredient entering the rumen like soybean meal, corn gluten feed, etc. Tofu dregs, a main by-product from tofu making, is considered as a high quality feed ingredient and it is commonly used as a protein source for ruminants, particularly in an area or a nearby area where tofu is produced. Protein protection of such high quality feed ingredients from microbial degradation in the rumen by using certain compounds may be needed in order to maintain the quality of feed protein.
Tannin is a polyphenolic compound from the group of plant secondary metabolites with the ability to bind and precipitate protein (Kondo et al., 2014; Jayanegara et al., 2019). It is part of plant defense mechanism to deter attack from various organisms such as herbivores, parasites, fungi, insects, and animals (Waghorn, 2008). Tannin presents in different parts of a plant such as in the roots, stems, skin, leaves and fruits (Hussein, 2017). Although tannin may reduce feed palatability and digestibility, it may provide beneficial effects on ruminant metabolism and productivity when used at an appropriate dosage (Frutos et al., 2004). Tannin can protect protein from degradation process by rumen microbes and therefore serves as a protein bypass agent (Deaville et al., 2010). Further, tannin-protein binding minimizes deamination process and thus increases the availability of amino acids in post-rumen organs (Orlandi et al., 2015). Other beneficial effects of tannin, apart from improving livestock productivity, are reducing the population of intestinal parasites, mitigating enteric methane emission, and elevating beneficial fatty acid profiles in animal products (Jayanegara et al., 2019).
Tannin in ruminant diets may be originated from forage containing tannin or from tannin extract as a feed additive. Some plants that have been commonly used as tannin sources are quebracho, mimosa and chestnut (Jayanegara et al., 2015). Acacia plant had been reported to contain considerable amount of tannin in its leaves (Jayanegara et al., 2011), but to date limited studies have explored tannin in the bark of acacia. This experiment aimed to extract tannin from acacia bark by using a hot water extraction system at different temperature and pressure. Further, the tannin extract was used as an additive for protecting tofu dregs from microbial degradation in an in vitro rumen fermentation system.
MATERIALS AND METHODS
Tannin extraction
Acacia bark was collected from acacia plantation around Bogor area, Indonesia. The bark was cut uniformly with a size of 0.5 cm × 1 cm, and then was put into a hot water extraction system. The extraction process was set two different temperatures (70 and 120oC) and two different pressure (1 and 2 bar), performed for 2 h period. Tannin was extracted and migrated from the bark into the water. The water was then determined for its total phenol, total tannin, condensed tannin and hydrolysable tannin by using a UV-Vis spectrophotometry method according to Makkar (2003). Temperature and pressure that extracted the highest tannin recovery were chosen for further steps. Extracted tannin was dried by using spray drying method and subsequently used as a feed additive for protecting tofu dregs from microbial degradation.
In vitro incubation procedure
Tannin was added to tofu dregs at various levels, i.e., tofu dregs without tannin (T0), tofu dregs + 1% acacia tannin (T1), tofu dregs + 2% acacia tannin (T2), tofu dregs + 3% acacia tannin (T3), and tofu dregs + 4% acacia tannin (T4). Tofu dregs was obtained freshly from a tofu company in Ciampea district, Bogor, Indonesia. It was oven-dried at 60oC for 24 h prior to mix with the acacia tannin extract. Samples were then incubated in vitro with rumen fluid and buffer mixture according to Theodorou et al. (1994). The rumen inoculum was obtained from two fistulated Ongole crossbred cattle in which the cattle were maintained according to the animal welfare standard of Indonesian Institute of Sciences. Approximately 500 mg sample was inserted into a 125 ml serum bottle and added with rumen fluid and buffer mixture, each of 15 and 60 ml, respectively. Allocation of treatments into the incubation bottles was according to a randomized complete design. Incubation was carried out in four replicates (four bottles per replicate). Serum bottles were then immediately sealed with butyl rubber stoppers and aluminum crimp seals. Incubation was carried out for 48 h at 39ºC. Gas production was recorded during the incubation. After 48 h, the supernatant was collected for determination of total volatile fatty acids (VFA) and ammonia concentration (Jayanegara et al., 2016). Residue was weighed to determine in vitro dry matter digestibility (IVDMD) and in vitro organic matter digestibility (IVOMD).
Data analysis
Data on gas production were fitted with the following non-linear regression model:
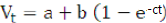
Where; Vt is the volume of gas production (ml) at time t, t is incubation period (h), a + b is the gas production potential, e is the natural number, and c is gas production rate constant. The goodness of fit of the model was evaluated through coefficient of determination (R2) and mean square error (MSE). Analysis of variance (ANOVA) was performed on all variables and continued with Duncan’s multiple range test when there was a significant difference among treatments at P<0.05.
Table 1: Effect of temperature and pressure on phenol and tannin contents (%DM) of acacia bark extracted by hot water
| Parameter | Phenolic fraction | |||||||
| Treatment | Phenol | Tannin | T/P (%) | CT | HT | CT/T (%) | CT/HT | |
|
Temperature (oC) |
70 | 3.70 | 3.32 | 89.71 | 1.77 | 1.56 | 52.91 | 1.11 |
| 120 | 6.03 | 5.49 | 91.06 | 2.38 | 3.12 | 43.30 | 0.72 | |
| Pressure (bar) | 1 | 4.74 | 4.25 | 89.46 | 1.80 | 2.45 | 43.77 | 0.80 |
| 2 | 5.24 | 4.81 | 91.63 | 2.47 | 2.35 | 51.34 | 1.02 | |
| SEM | 0.35 | 0.33 | 0.64 | 0.13 | 0.24 | 2.18 | 0.08 | |
| Significance | ||||||||
| Temperature | *** | *** | ns | *** | *** | *** | *** | |
| Pressure | ns | Ns | ns | *** | ns | *** | *** | |
| Temp*Pressure | ns | Ns | ns | ns | ns | ns | ns | |
Different superscripts within the same column are significantly different at P<0.05. T/P, tannin to phenol percentage; CT, condensed tannin; HT, hydrolysable tannin; CT/T, condensed tannin to tannin percentage; SEM, standard error of mean. ***, P<0.001; ns, non-significant.
RESULTS AND DISCUSSION
Phenol, tannin, CT and HT contents in acacia bark were higher (P<0.001) when extracted at 120oC as compared to that at 70oC (Table 1). Higher pressure did not result any different in the phenol and tannin contents of acacia bark. However, CT proportion was elevated by the higher pressure in comparison to the lower one (P<0.001). There was no interaction between temperature and pressure with regard to the ability to extract tannin. Therefore, the temperature of 120 oC and the pressure of 1 bar were chosen for extracting tannin from acacia bark and subsequently to use the tannin extract for protecting ruminal degradation of tofu dregs.
Effectiveness of tannin extraction on acacia bark depends on its extraction method. Tannin extraction from acacia bark by using water at room temperature for 24 h resulted total tannin and total phenol of 7.1 and 4.5%, respectively (Wina et al., 2010). These results were closely similar to the present experiment regarding the values of phenol and tannin. Extraction with 50% methanol or 50% acetone yielded tannin by 16.1 or 17.5%, respectively (Wina et al., 2010). In another study, extraction of acacia bark by 80% methanol at 60oC for 2 h resulted 16.6% tannin (Naima et al., 2015). Makkar (2003) recommended 70% acetone to optimally extract tannin from various plant materials since the compound has both polar and non-polar functional groups within its chemical structure. However, the use of organic solvent may not be economically feasible to extract tannin under a large scale condition for commercial purpose. Furthermore, disposal of organic solvent under such large scale may create problem to the environment if it is not properly managed. The use of water at an elevated temperature is apparently an optimal option to extract tannin from acacia bark for cost efficiency and environmentally friendly objectives.
Addition of tannin extract from acacia up to 2% generally did not decrease in vitro gas production of tofu dregs during the incubation period (Table 2). The addition of tannin at 3 and 4% reduced the gas production of tofu dregs right from the early incubation period to the end in comparison to that of control treatment (P<0.05). With regard to gas production kinetic parameters, addition of tannin extracts up to 4% did not alter the gas production potential (Table 3). However, gas production rate was reduced when tofu dregs was added with tannin extract at 3 and 4% as compared to that of control (P<0.05). The non-linear equation produced a high goodness of fit as indicated by the high R2 and the low MSE. Total VFA and ammonia concentrations were reduced by 19.9 and 58.6% (P<0.05), respectively, in the addition of 1% tannin extract (Table 4). Higher levels of tannin additions led to further decrease of these parameters. Similarly, IVDMD and IVOMD of tofu dregs were reduced due to acacia tannin extract addition at 1 to 4% (P<0.05). Treatments of T3 and T4 resulted in lower IVDMD and IVOMD values than those of T1 and T2 (P<0.05).
The reduction of gas production in the addition of tannin extract from acacia bark is apparently due to a decrease in nutrient degradability of tofu dregs. This is confirmed by the reduction of IVDMD and IVOMD following the tannin extract addition. Gas production has been well-known to be positively correlated with nutrient degradability in the rumen (Kamalak et al., 2005). Among the nutrients, carbohydrate both structural and non-structural contributes most to the gas production in the in vitro rumen fermentation system, whereas lipid produces only negligible amount of gas. Tannin has the affinity to interact with carbohydrate thus preventing it from microbial degradation in the rumen (Ahnert et al., 2014), and as a consequence, produces lower amount of gas. Apart from the interaction between tannin and nutrients, certain population of bacteria and digestive enzyme activity are inhibited in the presence of tannin (Ogawa and Yazaki, 2018). For instance, condensed tannin from Vaccinium vitis idaea had the ability to reduce total bacteria and some fiber degrading bacteria such as Butyrivibrio fibrisolvens and Butyrivibrio proteoclasticus (Szczechowiak et al., 2016). In another study, Jayanegara et al. (2015) demonstrated that both condensed and hydrolysable tannins decreased the population of Fibrobacter succinogenes and Ruminococcus flavefaciens. Such depression in fiber digestion is related to the VFA decrease since the latter is a final product from the former. The VFA concentration in the present study was within the normal range from 70 to 130 mmol/l (Dijkstra et al., 2005).
Table 2: In vitro gas production (ml) of tofu dregs added with acacia tannin extract.
| Treatment | Incubation period (h) | |||||||
| 2 | 4 | 6 | 8 | 10 | 12 | 24 | 48 | |
| T0 |
12.0bc |
24.5bc |
42.6c |
62.0c |
77.1c |
89.5c |
121.9c |
141.6b |
| T1 |
12.9bc |
26.6c |
45.6d |
65.9d |
80.8d |
92.3d |
123.9c |
140.0b |
| T2 |
13.6c |
26.4c |
43.6c |
62.6c |
77.1c |
88.8c |
122.1c |
143.4c |
| T3 |
11.5b |
23.0b |
38.9b |
56.6b |
70.4b |
82.3b |
115.3b |
137.9a |
| T4 |
10.3a |
20.8a |
35.3a |
51.7a |
64.3a |
75.8a |
110.6a |
135.5a |
| SEM | 0.27 | 0.53 | 0.90 | 1.23 | 1.41 | 1.42 | 1.24 | 0.78 |
| Sign | *** | *** | *** | *** | *** | *** | *** | *** |
Different superscripts within the same column are significantly different at P<0.05. T0, tofu dregs (control); T1, tofu dregs + 1% acacia tannin; T2, tofu dregs + 2% acacia tannin; T3, tofu dregs + 3% acacia tannin; T4, tofu dregs + 4% acacia tannin; SEM, standard error of mean; Sign, significance; ***, P<0.001.
Table 3: In vitro gas production kinetic parameters of tofu dregs added with acacia tannin extract.
| Treatment | a+b (ml) | c (/h) | R2 | MSE |
| T0 |
148.2ab |
0.073c |
0.99 | 33.0 |
| T1 |
147.9ab |
0.077d |
0.99 | 32.6 |
| T2 |
149.8b |
0.072c |
0.99 | 23.5 |
| T3 |
145.6a |
0.066b |
0.99 | 23.3 |
| T4 |
145.6a |
0.059a |
0.99 | 18.3 |
| SEM | 0.54 | 0.003 | ||
| Sign | * | *** |
Different superscripts within the same column are significantly different at P<0.05. T0, tofu dregs (control); T1, tofu dregs + 1% acacia tannin; T2, tofu dregs + 2% acacia tannin; T3, tofu dregs + 3% acacia tannin; T4, tofu dregs + 4% acacia tannin; SEM, standard error of mean; Sign, significance; ***, P<0.001; *, P<0.05; ns, non-significant.
Ammonia decrease after tannin addition indicates that the compound binds protein present in tofu dregs. In the rumen, protein is degraded to various amino acids and subsequently fermented to α-keto acid and ammonia. Therefore, ammonia concentration reflects the extent of protein degradation by rumen microbes. Tannin has a strong affinity on protein and able to precipitate the nutrient in aqueous solutions (Silanikove et al., 2001). Through such interaction, rumen microbes are hindered to degrade protein in the presence of tannin. This result is in agreement with other studies that showed the negative relationship between tannin and ruminal ammonia concentration (Kondo et al., 2014). Protection by tannin is expected to by-pass the protein through the rumen and later on is utilized by livestock in post-rumen organs (Deaville et al., 2010). The ability of tannin to inhibit proteolysis does not only occur in the rumen but also under anaerobic condition during silage making. The study of Jayanegara et al. (2019) revealed that tannin negatively affected soluble nitrogen, free amino acid nitrogen, non-protein nitrogen and ammonia concentrations in silage.
Table 4: In vitro ruminal fermentation parameters of tofu dregs added with acacia tannin extract.
| Treatment | VFA (mmol/l) | NH3 (mmol/l) | IVDMD (%) | IVOMD (%) |
| T0 |
118.8c |
36.2c |
72.0c |
68.3c |
| T1 |
95.2b |
15.0b |
68.4b |
60.1b |
| T2 |
72.9a |
12.7ab |
67.7b |
57.7b |
| T3 |
75.5a |
12.6ab |
64.7a |
47.2a |
| T4 |
72.9a |
11.0a |
64.3a |
46.0a |
| SEM | 2.33 | 4.63 | 0.68 | 1.53 |
| Sign | *** | *** | *** | *** |
Different superscripts within the same column are significantly different at P<0.05. T0, tofu dregs (control); T1, tofu dregs + 1% acacia tannin; T2, tofu dregs + 2% acacia tannin; T3, tofu dregs + 3% acacia tannin; T4, tofu dregs + 4% acacia tannin; SEM, standard error of mean; Sign, significance; ***, P<0.001.
CONCLUSION
Extraction of tannin from acacia bark by using a hot water extraction system is optimal when conducted at 120oC at 1 bar for 2 h. Acacia tannin is able to protect microbial degradation of tofu dregs in the rumen environment as indicated by the lower gas production, total VFA, ammonia, IVDMD and IVOMD values. With special reference to protein breakdown in the rumen, acacia tannin may strategically be used to protect the component thus serves as a protein by-pass agent.
ACKNOWLEDGEMENT
The authors are grateful to Ministry of Education and Culture (KEMDIKBUD), Republic of Indonesia, for funding this research through “Penelitian Dasar - Penelitian Berbasis Kompetensi” grant, year 2020.
AUTHORS CONTRIBUTION
TUPS carried out the experiment, performed the statistical analysis and wrote the initial manuscript draft. RR, N and AJ designed and supervised the experiment and revised the manuscript.
CONFLICT OF INTEREST
All authors have declared no conflict of interest.
References





