Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Haemoprotozoan Diseases in Cattle Population in Sylhet District of Bangladesh
Md. Motaleb Hosen1, Abul Bashar Jewel1, Md. Shahidur Rahman Chowdhury1, Md. Bashir Uddin1, Md. Mukter Hossain1, Md. Masudur Rahman2, Md. Mahfujur Rahman1*
1Department of Medicine, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet, 3100, Bangladesh; 2Department of Pathology, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet, 3100, Bangladesh.
Abstract | This study aimed to conduct a cross-sectional study to investigate the prevalence of haemoprotozoan diseases in Sylhet district of Bangladesh. A one year (January to December 2018) study on hemoprotozoan diseases was conducted in crossbred and indigenous cattle. Blood samples were collected randomly from 81 crossbred and from 19 indigenous cattle from four representative areas in three seasons. Blood samples were examined by Giemsa’s stained thin blood smear method. The effect of breed, sex, age and season was observed in cattle during this study. The overall prevalence of haemoprotozoan diseases in Sylhet district was 52%. Three (3) types of haemoprotozoan diseases have been identified (Anaplasmosis, Babesiosis, Mixed) among them prevalence of Anaplasmosis was 28%, babesiosis was 08% and mixed infection was 15%. The prevalence of haemoprotozoan diseases was not significant (P>0.05) in relation to breed but the highest prevalence found in crossbreed cattle was Anaplasmosis (29.63%). Sex-wise prevalence was also not significant (P<0.05) in each of the diseases and here, the highest prevalence was found in male (31.48%) in case of Anaplasmosis. In relation to age, only mixed infected cattle were differ significantly (P<0.05) where the highest prevalence was observed (30.43%) in case of Anaplasmosis. Hemoprotozoan diseases were predominant in summer (36.11%) season followed by rainy (29.41%) and winter (16.67%) season. In case of mixed infection, adult cattle had significantly higher prevalence which was statistically significant (P<0.05). Study results revealed that burden of haemoprotozoan diseases are apparently high in Sylhet district regardless of the age, sex, breed and season. The data generated through this study will help to take adoptive control measures against haemoprotozoan diseases in Bangladesh.
Keywords | Prevalence, Haemoprotozoan diseases, Cattle, Sylhet, Bangladesh
Received | March 28, 2020; Accepted | June 6, 2020; Published | June 25, 2020
*Correspondence | Md. Mahfujur Rahman, Associate Professor, Department of Medicine, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet, 3100, Bangladesh; Email: mahfuj.vetmed@sau.ac.bd
Citation | Hosen MM, Jewel AB, Chowdhury MSR, Uddin MB, Hossain MM, Rahman MM, Rahman MM (2020). Prevalence of haemoprotozoan diseases in cattle population in Sylhet District of Bangladesh. Adv. Anim. Vet. Sci. 8(7): 748-752.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.7.748.752
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Hosen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Haemoprotozoan diseases cause devastating losses to the livestock industry in tropical and subtropical regions of the World (Velusamy et al., 2014). Most of the haemoprotozoan parasites are tick borne and they have significant importance to the world animal health (Uilenberg, 1995). Tick-borne haemoprotozoan diseases cause major losses in production of meat and milk and is of great economic importance in Asia and has always been a formidable barrier to the survival of exotic and cross bred cattle in Bangladesh. The hot and humid climate of Bangladesh is favorable for growth, multiplication and survival of arthropods, which serve as vector for many blood-borne protozoan diseases (Krishnamurti et al., 2016).
Eighty percent (80%) of the rural people of Bangladesh rear indigenous cattle (Siddiki et al., 2010) and many of them are dependable on dairy farming under the traditional husbandry practices. The haemoprotozoan diseases greatly hampered the health and productivity of cattle (Malayar and Farid, 2018) among which Babesiosis, Anaplasmosis and Theileriosis are considered the major haemoprozoan diseases in Bangladesh (Rajput et al., 2005). Control of the haemoprotozoan diseases costs a lot and livestock industry has to face great obstacles to introduce genetically improved cattle in a specific area (Makala et al., 2003; Radostits et al., 2000).
Several blood protozoa have been reported in animals of Bangladesh including Babesia, and Theileria (Mahmud et al., 2015). The topography of Sylhet district of Bangladesh is much diversified which comprises plane, semi-hilly and hilly areas. There is no study for investigating the haemoprotozoan diseases in Sylhet district of Bangladesh, especially in hilly and semi-hilly areas. On the other hand, the climatic condition and geographical location of these areas might favour the growth and multiplication of different vectors. Hence, an attempt was made to study the prevalence of haemoprotozoan diseases in cattle population in Sylhet district of Bangladesh.
MATERIALS AND METHODS
Study area and population
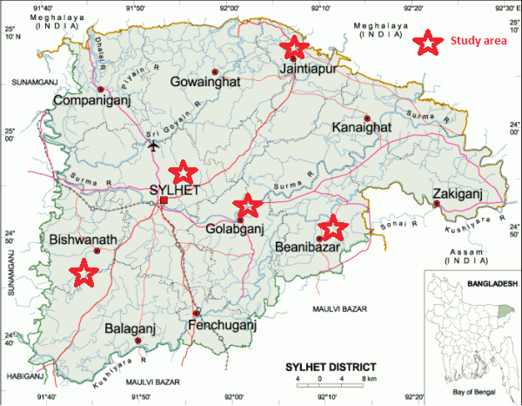
The study was conducted at Sylhet Sadar, Jaintapur, Bishwanath, Beanibazar and Golapgonj Upazilla of Sylhet district (Figure 1) for a period of 12 months from January to December 2018. Holstein Friesian (HF) crossbred (Bos taurus X Bos indicus) and indigenous cattle (Bos indicus) were selected for this study as target animals. A total of 100 cattle aged from <1year, 1-2 year and > 2 years of either sex (male 54 and female 46) were randomly selected from villages in Sylhet district of Bangladesh. As three seasons of Bangladesh are prominent such as summer (March- June), rainy (July-October), winter (November-February), the sampling was performed in three seasons.
Collection and microscopic examination of blood samples
Approximately 3-5 ml of blood sample was collected from jugular vein using 10 ml disposable plastic syringe from each animal and then preserved in BD Vacutainer® tube containing anticoagulant (Lithium Heparin). The collected blood samples were brought to the laboratory-1, in the Department of Medicine, Faculty of Veterinary, Animal and Biomedical Sciences (FVABS), Sylhet Agricultural University, Sylhet (SAU), in insulated carry box containing ice packs to maintain low (4 °C) temperature and then the samples were kept in the refrigerator at 4°C for further processing. Further examination was done by preparing two thin smears from each blood sample (Hendrix and Robinson, 2006) and subsequently stained with Giemsa’s stain. Fifty fields from each stained slides were examined under binocular microscope (X 100) for identification of blood protozoa at genus level (Urquhart et al., 1996).
Statistical analysis
Collected data were arranged in MS Office Excel and Statistical analysis was carried out by Chi square (χ2) test using Statistical Package for Social Science (SPSS) version 20. For Chi-Square Test, results were expressed in percentage with P-value and significance was determined when P<0.05.
RESULTS
In the present study, a total of 100 blood smears from 100 cattle were examined, out of which 52 animals were found to be positive for haemoprotozoan infection. The overall prevalence of haemoprotozoan diseases was 52% (Table 1). Among 100 blood smears examined, 28% cattle were infected with Anaplasmosis, 09% with Babesiosisand 15% were mixed infection (Table 1). No case of Theileriosis wasfound in this study.Prevalence of haemoprotozoan diseases in cattle washigher in cross breed cattle than indigenous cattlebut it was not statistically significant (P<0.05). Both the prevalence of Anaplasmosis and Babesiosis were higher in cross breed cattle but in case of mixed infection the prevalence was higher in indigenous cattle (Table 2).
Table 1: Overall prevalence of haemoprotozoan diseases in Sylhet district.
| Diseases | No. of affected animal N=100 | Prevalence (%) |
| Anaplasmosis | 28 | 28 |
| Babesiosis | 09 | 09 |
| Mixed | 15 | 15 |
| Total | 52 | 52 |
Table 2: Breed wise prevalence of haemoprotozoan diseases in cattle in Sylhet district.
| Variable | Category | N | Babesiosis | Anaplasmosis | Mixed | |||
| % | P-value | % | P-value | % | P-value | |||
| Breed | Cross | 81 | 9.88 | 1.000 | 29.63 | 0.454 | 13.54 | 0.476 |
| Indigenous | 19 | 5.26 | 21.05 | 15.79 | ||||
N: No. of animals; %: Percentage; *: Significant (P<0.05).
The prevalence of haemoprotozoan diseases was not differing significantly in relation to sex (Table 3). In this study, it was found that the prevalence of both the Anaplasmosis (31.48%) and mixed infection (18.52%) werehigher in malewhereas the prevalence of Babesiosis was higher (13.04%) in female. Among the haemoprotozoan diseases, Anaplasmosis had the higher prevalence in case of both male and female.
Table 3: Sex wise prevalence of haemoprotozoan diseases in cattle in Sylhet district.
| Variable | Category | N | Babesiosis | Anaplasmosis | Mixed | |||
| % | P-value | % | P-value | % | P-value | |||
| Sex | Male | 54 | 5.54 | 0.294 | 31.48 | 0.401 | 18.52 | 0.286 |
| Female | 46 | 13.04 | 23.91 | 10.87 | ||||
N: No. of animals; %: Percentage; *: Significant (P<0.05).
Table 4: Age wise prevalence of haemoprotozoan diseases in cattle in Sylhet district.
| Variable | Category | N | Babesiosis | Anaplasmosis | Mixed | |||
| % | P-value | % | P-value | % | P-value | |||
| Age | Calf | 7 | 0.00 | 0.116 | 28.57 | 0.665 | 0.00 |
0.015* |
| Young | 24 | 0.00 | 20.83 | 0.00 | ||||
| Adult | 69 | 13.03 | 30.43 | 21.74 | ||||
N: No. of animals; %: Percentage; *: Significant (P<0.05).
The animals were divided into three age groups (calf, young, adult) according to their age. The mixed infected cattle were differ significantly (P<0.05) in relation to age. But like sex-wise variation, here, the prevalence of Anaplasmosis was also higher in adult than the other haemoprotozoan infections. Interestingly, no case of Babesiosis was observed in case of calf and young cattle.
As shown in Table 5, the highest prevalence in case of all categories of diseases was recorded in rainy season and the lowest prevalence was recorded in winter season. Among the diseases the highest prevalence was observed in case of Anaplasmosis (36.11%) in summer and lowest prevalence was observed in case of Babesiosis and mixed infection (3.33% in both case). The seasonal prevalence of Anaplasomosis and Babesiosis did not differ significantly (P>0.05) however, it was significant only in case of mixed infection (P<0.05).
Table 5: Season wise prevalence of haemoprotozoan diseases in cattle in Sylhet district.
| Variable | Category | N | Babesiosis | Anaplasmosis | Mixed | |||
| % | P-value | % | P-value | % | P-value | |||
| Season | Winter | 30 | 3.33 | 0.340 | 16.67 | 0.210 | 3.33 |
0.034*
|
| Summer | 36 | 8.33 | 36.11 | 13.89 | ||||
| Rainy | 34 | 14.71 | 29.41 | 26.47 | ||||
N: No. of animals; %: Percentage; *: Significant (P<0.05).
DISCUSSION
Overall prevalence
The overall prevalence of hemoprotozoan diseases in our study is 52%, which contradicted with the reports of Kamani et al. (2010) in Nigeria (25.9%) and Ananda et al., (2009) in Bangalore North, India (43.18%). These differences observed in the prevalence might be due to the difference in geographical locations of the studies, time periods and various methods of sample analysis.
Breed-wise prevalence
In case of Babesiosis, the highest prevalence was reported in cross breed cattle (9.88%) followed by indigenous cattle (5.26%) but in case of mixed infection prevalence was higher in indigenous cattle than cross breed cattle. This case of high infection in indigenous cattle might be due to the fact that indigenous cattle are more exposed to tick (Ghafar et al., 2011). In the study area it was observed that the indigenous cattle were moved freely in pasture land whereas crossbreed cattle were confined to farms. The higher prevalence of babesiosis in crossbreed cattle in our study is in line with the findings of Alim et al. (2012). In the present study, higher prevalence of Anaplasmosis in crossbreed cattle was observed as compared to indigenous cattle. Our findings are in correspondence with the findings of Chowdhury et al. (2006) who recorded prevalence of anaplasmosis in cross breed cattle (58.33%) and indigenous cattle (11.63%) in Sirajganj district. Other studies also reported the high herd seropositivity of Anaplasmosis (Urdaz-Rodríguez et al., 2009). Breed differences are also important in the susceptibility of cattle to tick borne diseases where Cattle of European origin like Holstein are usually highly susceptible (Tabor et al., 2017). Breed susceptibility of Babesiosis recorded in this study was supported by the report of Chakraborti (2002). Variation in geo-climatic condition, breed, and exposure of vectors and age of the animals might contribute to variable prevalence of hemoprotozoan diseases in the study areas (Muhanguzi et al., 2010). Constant exposure of infections and development of immunity against such infections might be responsible for lower prevalence in indigenous cattle (Siddiki et al., 2010). On the contrary, more attention in the management of HF crossbred cattle might give less chance of pre exposure of vectors and develop no or less immunity, resulting frequent occurrence of such diseases (Chowdhury et al., 2006; Ananda et al., 2009; Siddiki et al., 2010).
Sex-wise prevalence
In current study higher prevalence of Babesiosis was observed in female cattle (13.04%) compared to male cattle (5.54%). The findings of our study are in agreement with the findings of Belal et al. (2014) who also reported higher prevalence of anaplasmosis in female cattle (2.59%) than male cattle (1.60%). One possible explanation of getting higher cases of babesiosis in female cattle might be due to the fact that they were kept longer for breeding and milk production purpose, supplied insufficient feed against their high demand (Kamani et al., 2010) or variation in sample size. On the other hand in case of anaplasmosis the trend showed an opposite direction. In that case high prevalence of theileriosis observed in male cattle (31.48%) compared to their female counterparts (23.91%).
Age-wise prevalence
In our present study adult cattle had the highest prevalence of Babesiosis and Anaplasmosis (13.03% and 30.43% respectively). To our astonishment, we could not observe a single case of Babesiosis in young cattle and calf. In similar works on anaplasmosis implemented in Sirajganj district by Belal et al. (2014), higher prevalence was found in adult cattle (34.19%) followed by young cattle (20.51%), and calf (14.11%). Observation of this study also supported by the findings of Kamani et al. (2010) who observed higher prevalence in adult than young cattle. Findings of babesiosis in this investigation were supported by the observations of Taylor et al. (2015) and Zintl et al. (2005) who reported an inverse age resistance of the disease where adult showed more susceptibility than calves. This might be due to rapid immune responses to primary infection by the calves through a complex immune mechanism (Zintl et al., 2005).
Prevalence of anaplasmosis in this study supported the reports of Chakraborti (2002) and Chowdhury et al. (2006) who observed comparatively higher prevalence in adult than calves. Endemic instability of the study areas might be responsible for frequent infections in adult cattle where newborn calves were protected by colostral immunity (Cynthia et al., 2011). On the contrary, earlier observation was in contrast with the observation of Muhanguzi et al. (2010) who found higher prevalence of anaplasmosis in calves and lowest in young cattle in Uganda and the difference was explained by dominant immune responses to Anaplasma spp. infection. Age resistance, perhaps in combination in some cases with maternal antibodies, might be reflected in the reduced number of clinical outbreaks in young animals.
Season-wise prevalence
In our study we observed a significant influence of season on the occurrence of hemoprotozoan infections (mixed cases, P= 0.034) in Cattle. In rainy season the prevalence of mixed infections was highest in cattle. The findings of our study demonstrated that rain had a significant effect on the prevalence of hemoprotozoan infections in cattle. This may be explained by the fact that the tick population is abundant during rainy seasons. In another study it was found that tick population were peak depending on temperature, humidity, and rainfall which might be accounted for higher prevalence of hemoprotozoan diseases in rainy season (Ananda et al., 2009; Sanjay et al., 2007) whereas lower temperature and humidity of winter season is less favorable for the growth and multiplication of tick vectors which might contribute to lower frequency of such diseases in the study population (Muhammad et al., 1999; Zahid et al., 2005).
CONCLUSION
The present study concluded that the Sylhet district of Bangladesh was highly endemic for Anaplasmosis, Babesiosis and Theileriosis. Prevalence of these diseases was higher during summer months due to the high prevalence of tick population. This study could be useful to forecast the diseases based on seasonality. Screening of carrier status is important for early diagnosis and implementation of tick control measures to prevent economic losses in cattle.
ACKNOWLEDGMENTS
The authors are highly thankful to the Department of Medicine, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet for providing the research facilities.
AUTHORS CONTRIBUTIONS
All authors contributed equally.
CONFLICT OF INTERESTS
The authors have declared no conflict of interests.
REFERENCES
Kamani J, Sannusi A, Egwu OK, Dogo GA, Tanko TJ, Kemza S, Tafarki AE, Gbise DS (2010). Prevalence and significance of haemoparasitic infections of cattle in North-Central, Nigeria. Vet. World. 3 (9): 445-448. https://doi.org/10.5455/vetworld.2010.445-448