Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Antibiotic Residues and Associated Risk Factors in Milk in Chittagong, Bangladesh
Md Saiful Bari1*, AKM Humayun Kober1, Md. Ahasanul Hoque2, Goutam Kumar Debnath1, Gouranga Ch. Chanda1
1Department of Dairy and Poultry Science, Faculty of Veterinary Medicine, Chattogram Veterinary and Animal Sciences University, Khulshi, Chattogram 4225, Bangladesh; 2Department of Medicine and Surgery, Faculty of Veterinary Medicine, Chattogram Veterinary and Animal Sciences University, Khulshi, Chattogram 4225, Bangladesh.
Abstract | We conducted the study to determine the prevalence and concentration of antibiotic residues (AR) in raw and pasteurized milk along with the effect of boiling on the concentrations as well as the associated risk factors on residues in raw milk. We collected 380 raw milk samples from commercial dairy farms, household farms and different distributing points, and 100 pasteurized milk samples from different markets of Chittagong, Bangladesh. A pretested questionnaire was applied to identify farm-level risk factors for AR followed by a univariate analysis to test the factors. The milk samples were screened for the presence of AR by Thin Layer Chromatography (TLC) and the concentration of residues (µg/l) was determined by Ultra High Performance Liquid Chromatography (UHPLC). Categories of farms, cow illness, treatment given in last week and antibiotics used in treatment were significant (P ≤ 0.05) factors for prevalence of AR in raw milk (18 %). The concentrations of amoxicillin (339.9 µg/l) and oxytetracycline (195 µg/l) residues in raw milk were significantly (P ≤ 0.01) reduced by boiling. The concentrations of amoxicillin and oxytetracycline residues in all sources of milk were higher than the Maximum Residue Limit (MRL) and assumed to be causing a serious public health threat.
Keywords | Antibiotic residue, Boiling, Prevalence, Milk, Risk factor
Received | April 07, 2020; Accepted | June 04, 2020; Published | June 08, 2020
*Correspondence | Md Saiful Bari, Assistant Professor, Department of Dairy and Poultry Science, Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong 4225, Bangladesh; Email: saifulbari@cvasu.ac.bd
Citation | Bari MS, Kober AKMH, Hoque MA, Debnath GK, Chanda, GC (2020). Prevalence of antibiotic residues and associated risk factors in milk in Chittagong, Bangladesh. Adv. Anim. Vet. Sci. 8(7): 701-708.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.7.701.708
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Bari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Antibiotics are used extensively in commercial dairy farms in South and South-East Asian countries including Bangladesh with the aim of preventive and therapeutic measures. Approximately 80% of all food-producing animals such as cattle, sheep, goats, and pigs receive antibiotics for part or most of their lives (Lee et al., 2001). Cattle is the major milk-producing animal in Bangladesh (Datta et al., 2019) and mastitis is the most prevalent disease of lactating cows (Hossain et al., 2016). Mastitis is the main impediment for dairy development and threat for dairy cows in Bangladesh (Kabir et al., 2017) and usually requires antibiotic treatment (Mohsenzadeh and Bahranipour, 2008). As field veterinarians are limited in Bangladesh, para vets and farmers also treat animals. Additionally, farmers can easily purchase drugs from shops in markets without a prescription. Thus, antibiotics are indiscriminately used in lactating cows without consulting veterinarians (Founou et al., 2016). Farmers or para vets and in some cases, veterinarians are unaware about antibiotic withdrawal periods. The presence of residues in milk may result from failure to maintain the mandatory withdrawal periods and over use of antibiotics (Kabir et al., 2004). Veterinarians or livestock professionals in Bangladesh typically do not encourage farmers to adhere to the drug withdrawal period for food producing animals. Therefore, farmers are not motivated enough and their lack of knowledge about the persistence of drug residues in milk, consequently results in AR in milk that can easily affect humans through consumption and human pathogens can become drug resistant (Lohren et al., 2009).The lack of awareness by the farmers, veterinarians, para-vets and others livestock professionals about the proper use and dose of antibiotics including maintaining withdrawal periods are contributing factors for AR contamination in milk (Mahmoudi et al., 2014). In addition, current use of antibiotics on-farms, cows under treatment in the past week, and number of cows presently sick or sick within the last week might be crucial factors for AR contamination in milk. It is of utmost importance to know the level of AR in milk and associated risk factors to be able to take necessary actions for minimizing the prevalence of AR in milk and reduce public health hazards.
Antibiotic residues in both raw and pasteurized milk have been investigated across the world. In Bangladesh, the prevalence of Amoxicillin and oxytetracycline was determined in 23 % and 38 % of milk samples, respectively (Chowdhury et al., 2015). The reported prevalence estimates of beta lactam AR in raw milk and market milk (pasteurized) in Iran were 6 % to 36 % (Khaskheli et al., 2008) and 3% to 21 % (Movassagh and Karami, 2010) respectively; and cefaprin was 3.8 % in raw milk (Ghidini et al., 2002) in Italy. The concentration levels of AR in raw milk in Iran were reported to be 8.5 µg/l to 53.7 µg/l for amoxicillin, 5.7 µg/l to 6.4 µg/l for cefaprin (Ghidini et al., 2002), and 150.4 µg/l for oxytetracycline. In market milk, AR concentrations were reported to be 87.1 µg/l for beta lactams (Abbasi et al., 2011) and the prevalence of AR was 24.8 % as recorded in Iran (Moghadam et al., 2016).
In developing countries such as Bangladesh, a good number of people are still unaware of milk-borne diseases and they drink raw milk, but some people consume milk after boiling instead of buying pasteurized milk from the market. Some antibiotics such as amoxicillin, oxytetracycline and ceftriaxone are heat labile, whereas gentamicin, sulphadimidine and ciprofloxacin are heat stable (Heshmati, 2015; Thamthaweechok et al., 2018). Hence, boiling might have an effect on the concentration of AR in milk, but, there is currently limited available information in this regard.
Although veterinary drugs are widely used in dairy production in Bangladesh, to the best of the author’s knowledge there are few published scientific reports (Chowdhury et al., 2015) on the level of AR in milk with a limited number of antibiotics. The effect of boiling on the level of AR in milk is poorly understood. The risk factors associated with the prevalence of AR in milk are still untouched. Hence, the current study was conducted to determine the prevalence and concentrations of AR in milk of Chittagong, Bangladesh along with the effect of boiling on residue concentrations. We predicted that some milk samples would have AR contamination and there would be variation in the prevalence of AR in milk of different sources. We also investigated the potential risk factors for AR contamination in milk.
MATERIALS AND METHODS
Ethical approval
The current study was approved by the Institutional Animal Ethics Committee with regards to its experimentation and the procedures used (Approval no: EC/2014/34-7).
Cross-sectional study
Study area
From November 2014 – October 2015, pasteurized milk samples were collected from different outlets from Chittagong, Bangladesh. The raw milk samples and farm information was obtained from Chittagong as dairy farming is densely established in both the Chittagong Metropolitan Area (CMA) and Patiya Upazilla of Chittagong.
Sample size calculation
In case of commercial dairy farms, a total of 100 farms in CMA were required assuming 38 % expected prevalence of AR (regardless of types), 0.05 ± precision, 95 % confidence interval, 1 % design effect and with a total number of 136 commercial farms. For the household farms, a total of 60 household farms was required assuming 14 % expected prevalence of AR (regardless of types), 0.05 ± precision, 95 % confidence interval, 1 % design effect and with a total number of 88 household farms (OpenEpi statistical software online. http://www.openepi.com/Menu/OE_Menu.htm, accessed: 16/06/2014).
Sample collection
A total number of 100 commercial dairy farms (≥ 3 cows) were selected randomly and 1 bulk milk sample was collected from each farm. Approximately 100ml of milk was obtained as a bulk milk sample from the tank where milk from all the cows was mixed and stored together. Also, a range of 1–5 individual milk samples were obtained from randomly selected cows per farm totaling 180 individual milk samples. Sixty household farms (< 3cows) were also randomly selected in Sikalbaha of Patiya upazila. One milk sample (approximately 100 ml) per household was collected. Forty milk samples (100ml) were collected from four milk distributing points (Sholasohor, Janalir hat, Karnafuli bridge and Potenga), each point contributed 10 samples. Every sample was collected from each point at two day intervals to avoid duplication as the wholesalers sometimes store milk for 2 days. One hundred market milk samples (approximately 100 ml) were collected from Brand A, Brand B, Brand C, Brand D and Brand E, each brand contributed 20 samples. Every sample was collected from each brand at a weekly interval because every brands renew their milk in the market every 7 days. Each sample was given a unique identification number before transporting to the laboratory on ice and stored at - 20 ° C until further analysis.
Data collection
The objectives of the study were thoroughly explained to the farmers and other participants and their consents were taken before interviewing and data collection. Field data were recorded through physical inspection and personal interviews using a pretested questionnaire. Farm size, disease presence, antibiotic treatment given during the last week (yes/ no), types of antibiotic use and other drug details (dose, route and knowledge about drug withdrawal period (yes/ no)) were recorded from commercial and household farms.
Laboratory evaluation
Sample processing and extraction
In order to precipitate the protein in the milk samples, 1ml of mixture of acetonitrile-methanol-deionized water (40 : 20 : 20) was added to 1ml of milk in a sterile falcon tube and mixed properly. This mixture was then centrifuged at 3000 rpm for 20 minutes and the supernatant was collected in an eppendorf tube for Thin Layer Chromatography (TLC) (Tyczkowska et al., 1989).
Thin layer chromatography (TLC)
TLC qualitatively detects type-specific AR. This TLC protocol was performed as described by Popelka et al. (2005). In this method, a standard solution of specific antibiotic along with each sample was pointed in the TLC plate and run at the same time in the mobile phase (Methanol : Acetone at 1 : 1). After 30 minutes the plates were taken out and dried. Then the plates were placed in a UV chamber and the Retardation Factor (RF) value was calculated for each sample. Almost equal lengths of RF of each standard antibiotic with experimental samples were considered positive for that antibiotic.
Ultra high performance liquid chromatography (HPLC)
Determination of amoxicillin and ciprofloxacin residues were quantified using the methods described by Wang et al. (2009), whereas oxytetracycline residue was quantified using the method established by Senyuva et al. (2000). Extracts positive to TLC were re-centrifuged for 15 minutes at 3000 rpm while contained in Eppendorf tubes followed by filtration via 0.2 µm MFS filters before using them for UHPLC evaluation. A stainless column C 18 (P/N 891 - 5002, 2 mm ID×10 0 mmL No. 22G2C - 001) was used for chromatography in all cases. The mobile phase was run at a flow rate of 0.2 ml/min, 1ml/min and 1.5 ml/min for amoxicillin, ciprofloxacin and oxytetracycline, respectively. In the case of amoxicillin and ciprofloxacin, the spectrometer wavelength was 254 nm, whereas it was 360 nm for oxytetracycline. Injection volume for amoxicillin and oxytetracycline was 20 µl but it was 10 µl for ciprofloxacin in the HPLC system.
Intervention study
Heat treatment on milk samples
An intervention trial was conducted in order to assess the effect of boiling on AR in milk. We boiled the TLC screened positive samples at 100 o C for 15 and 30 minutes. After the boiling treatment, the samples were again subjected to both TLC and UHPLC for screening for the presence of AR and determining the concentration of AR, respectively.
Statistical analysis
Data were checked and sorted in the MS Excel programme before exporting to STATA-11 (STATA Corp, USA). Descriptive analysis was performed on the results of AR according to different variables. Summary estimates were calculated on the concentration of AR. One-way ANOVA followed by a Bonferroni test was applied on the concentration of AR between treated (boiled) (15 and 30 minutes) and untreated groups.
A Chi-square test followed by a univariate logistic regression was performed to identify potential risk factors associated with a binary response variable of presence of AR of any type under the current investigation (yes/ no). The factors included for the analysis were commercial farm category such as small (3-25 cows), medium (26-50 cows) or large farms (more than 50 cows), illness of cows in the farm (yes/no), any antibiotic treatment given during the past week (yes/ no), and history of following a withdrawal period (yes/no). Farm categories were defined as per set criteria given by the Directorate of Livestock Services (DLS), Bangladesh. The level of significance (One-way ANOVA and Chi-square tests) was set at ≤ 0.05. The results of AR were presented in frequency numbers along with percentage according to categories of each variable, mean and standard error, odds ratio (OR), 95 % confidence interval and p value.
RESULTS
Prevalence of AR in milk and milk products
Among the milk samples, the prevalence of AR irrespective of antibiotic types was the highest (18 %) in the bulk samples from the commercial farms and the lowest (4 %) in the market milk (pasteurized) samples. The prevalence of AR was 9.4 % in case of individual cow samples from commercial farms, 8.3 % in household farm samples and 5 % in distributing point samples (Table 1).
From commercial farms bulk samples, the prevalence of AR for amoxicillin (4 %) and oxytetracycline (6 %), from individual commercial samples and household samples oxytetracycline (3.3 % and 5 %, respectively) were higher than the other types of antibiotics (Table 2). From distributing points, Karnafuli bridge’s milk samples had 10 % oxytetracycline and Potenga’s had 10 % ciprofloxacin residue positive. For market milk samples, 10 % of brand B milk for amoxicillin residue and 10 % of Brand D milk for oxytetracycline residue were confirmed (Table 2).
Association of risk factors with prevalence of AR in raw milk
The prevalence of AR was significantly (OR= 15.0, P= 0.03) higher in large farms (60 %) than medium farms (9.1 %). The farms that currently had sick cows had a significantly (OR= 11.3, P = 0.01) higher prevalence of AR (32 %) than the farms that had no diseased cows (4 %). The dairy farms with a history of ongoing treatment within the past week had a significantly (OR= 12.5, P= 0.01) higher prevalence of AR (33.3 %) in milk than the farms that had no such history (3.8 %) as presented in Table 3. The dairy farms that treated with antibiotics in the past week had a significantly (OR= 9.0, P= 0.00) higher (50 %) prevalence of AR in milk (50 %) than the farms without such treatment history (10 %) (Table 3).
Effect of heat treatment on AR in raw milk
After boiling for 15 minutes and 30 minutes, irrespective of types the AR percentage reduced to 16 % and 12 %, respectively from the prevalence 18% of bulk samples (P= 0.70). After boiling for 15 minutes and 30 minutes the AR positive percentages (9.4 %) of raw individual milk samples reduced to 8.9 % and 7.8 %, respectively. There were no significant differences (P= 0.81) in the percentage of AR among the raw, 15 minute-boiled and 30 minute-boiled milk samples from the commercial farms (P= 0.70), household farms (P = 0.88) and distributing points (P = 0.81) (Figure 1).
Effect of heat on concentrations of AR in raw milk
The concentrations of amoxicillin, oxytetracycline and ciprofloxacin in raw milk were 340 ± 13 µg/l, 195.0 ± 10 µg/l and 9.2 µg/l, respectively. Amoxicillin residue was in the highest and ciprofloxacin in the lowest concentrations in the raw milk samples. Among the market milk samples, the amoxicillin, oxytetracycline and ciprofloxacin residues were 132.9 µg/l, 78.3 µg/l and 0.6 µg/kg, respectively (Table 4). The highest concentrations (340 ± 13 µg/l) of amoxicillin residue in raw milk reduced to 258 ± 18 µg/l by 15 minutes of boiling and 120 ± 12 µg/l by 30 minutes of boiling (Table 4).
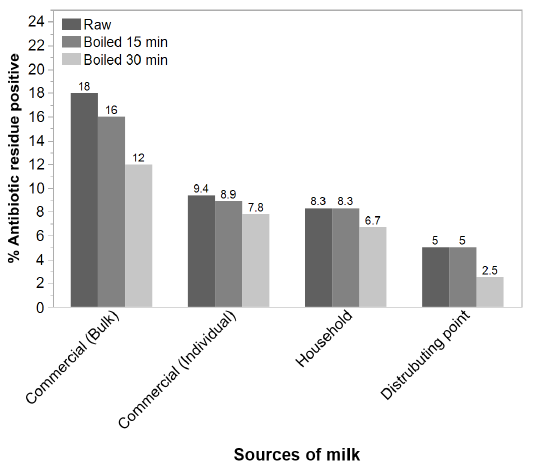
Figure 1: Effect of heat on prevalence of AR in milk of different sources. The sources of samples are indicated in the X-axis whereas the antibiotic residue % of samples are presented in the Y-axis. The color intensity of the bars indicates the state of the samples as (darker bar = raw milk samples, paler = 15 min boiled, pale = 30 min boiled samples).
There were highly significant differences (P<0.001) in amoxicillin residue concentrations in raw milk, 15 minute-boiled and 30 minute-boiled milk samples. The oxytetracycline residue in raw milk was 195.0 ± 10 µg/l. The concentration of oxytetracycline reduced in the 15 minute-boiled (100 ± 14 µg/l) and 30 minute-boiled milk samples (28±11 µg/l). The oxytetracycline residue concentration varied significantly (P < 0.001) among the raw milk, 15 minute-boiled and 30 minute-boiled milk samples (Table 4). The amoxicillin residue concentration varied significantly (P<0.001) between the raw milk and 30 minute-boiled milk samples and significant differences (P= 0.02) between the 15 minute-boiled and 30 minute-boiled milk samples. For oxytetracycline residue, the effect of heat was highly significant (P< 0.001) between the raw milk and 15 minute-boiled milk samples, the raw milk and 30 minute-boiled milk samples (P< 0.001) as well as the 15 minute-boiled and 30 minute-boiled milk samples (P< 0.001) (Table 4).
DISCUSSION
We evaluated the prevalence of antibiotic residues in raw, pasteurized and boiled milk along with the farm level risk
Table 1: Prevalence of antibiotic residues in milk of different sources.
| Source | Sample type | No of sample | % positive | 95% confidence interval |
| Commercial farm | Bulk milk | 100 | 18 | 0.09 – 0.31 |
| Individual | 180 | 9.4 | 0.06 – 0.15 | |
| Household farm | Individual | 60 | 8.3 | 0.01 – 0.17 |
| Distributing point | Individual | 40 | 5 | 0.00 – 0.45 |
| Market milk (Pasteurized) | Individual | 100 | 4 | 0.00 – 0.45 |
Table 2: Prevalence of different types of antibiotic residues in milk of different sources.
| Source | Sample type | N | AMX | OTC | GNT | CIP | CEF | SUL |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Commercial farm | Bulk milk | 100 | 4 (4) | 6 (6) | 4 (4) | 0 (0) | 2 (2) | 2 (2) |
| Individual | 180 | 2(1.1) | 6(3.3) | 5(2.8) | 0(0) | 3(1.7) | 1(0.6) | |
| Household | Individual | 60 | 0 (0) | 3 (5) | 2 (3.3) | 0 (0) | 0 (0) | 0 (0) |
| Distributing point | Sholasohor | 10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Karnafuli | 10 | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Jan Alir hat | 10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Potenga | 10 | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | |
|
Market milk (Pasteurized) |
Brand A | 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Brand B | 20 | 2(10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Brand C | 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Brand D | 20 | 0 (0) | 2(10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Brand E | 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Amoxicillin: AMX; OTC: Oxytetracycline; GNT: Gentamicin; CIP: Ciprofloxacin; CEF: Ceftriaxone; SUL: Sulphadimidine.
Table 3: Association of risk factors with prevalence of antibiotic residues in raw milk (Chi-square test, univariate logistic regression analysis).
| Variable | Category | Chi-square test | Univariate logistic regression | ||||
| AR (+ ve) n (%) | AR (-ve) n | P | OR | 95% CI | P | ||
| *Farm size | Small | 10 (14.7) | 58 | 0.033 | 1.72 | 0.18 – 16.59 | 0.634 |
| Medium | 2 (9.1) | 20 | 1.0 | - | - | ||
| Large | 6 (60.0) | 4 | 15.0 | 0.98 -228.9 | 0.029 | ||
| Cow sickness in last week | Yes | 16 (32.0) | 34 | 0.010 | 11.3 | 1.29 – 98.89 | 0.010 |
| No | 2 (4.0) | 48 | 1.0 | - | - | ||
| Treated in last week | Yes | 16 (33.3) | 32 | 0.007 | 12.5 | 1.43 – 109.65 | 0.007 |
| No | 2 (3.9) | 50 | 1.0 | - | - | ||
| Antimicrobials used last week | Yes | 10 (50.0) | 10 | 0.003 | 9.0 | 1.79 – 45.19 | 0.003 |
| No | 8 (10.0) | 72 | 1.0 | - | - | ||
*Small: 3 – 25 cows; Medium; 26 – 50 cows; Large = ≥ 51cows.
Table 4: Concentrations of antibiotic residues in raw milk (before and after heat treatment) and pasteurized milk.
| Sample type | Antimicrobial types | Concentration (µg/L) | ||||
| Before treatment | After treatment | Reference value | ||||
| 0 minute | 15 minutes | 30 minutes | P | |||
| Raw milk | Amoxicillin |
340±13a |
258±18a |
120±12b |
0.001 | 40 |
| Oxytetracycline |
195±10a |
100±14b |
28±11c |
0.001 | 100 | |
| Ciprofloxacin | 9.2 | 0.1 | 0.01 | - | 147 | |
| P – value | 0.001 | 0.002 | 0.010 | |||
| Pasteurized milk | Amoxicillin | 133 | - | - | - | 40 |
| Oxytetracycline | 78.3 | - | - | - | 100 | |
| Ciprofloxacin | 0.6 | - | - | - | 147 | |
a – c, Different superscript letters on the means in the same row indicate significant difference (P < 0.05).
factors for AR in raw milk. The commercial bulk samples had the highest AR prevalence. Farm size, history of cow sickness and antibiotic therapy were the significant risk factors. Boiling of milk samples significantly reduced the AR concentration in milk.
The prevalence of the AR was higher in the bulk samples from commercial dairy farms than individual samples. This was likely a result of the bulk samples being a mixture of milk from all the individual cows. oxytetracycline and gentamicin residues were determined in household milk. This might be due to the frequent use of oxytetracycline and gentamicin for treating cows in rural areas. The beta lactams and oxytetracycline were imprudently used in commercial dairy farms for treatment purposes, which coincided with the findings of Abebew et al. (2014) who determined oxytetracycline residues in milk within Ethiopia. The prevalence of AR in milk samples from distributing points were lower compared to other sources of milk as per the authors concerns this might have occurred because of the mixing of milk from different farms and consequently residue dilution. But, Manafi et al. (2010) and Mahmoudi et al. (2014) disagreed with the present results and recorded a higher prevalence of AR in milk collection points in Iran.
The market milk samples had the lowest percentage of AR. The finding was supported by previous studies (Mohsenzadeh and Bahranipour, 2008; Fonesca et al., 2009). But, Mahmoudi et al. (2014) reported higher prevalence in Iran than the present study. But, Movassagh and Karami, (2010) stated a slightly lower prevalence of AR in market milk in the Northeast region of Iran. The present finding was lower than the results of Aning et al. (2007) who conducted the investigation of AR in pasteurized milk in Ghana. The variation might be due to application of a different range of temperatures during pasteurization of milk in different plants and also regional variation in terms of cow sickness and use of antibiotics. This variation is also likely to be a result of the market milk being collected from different areas, then standardized and pasteurized (heat treated) simultaneously.
According to the present study, the milk samples from commercial farms contained AR of amoxicillin, oxytetracycline, ceftriaxone, gentamicin and sulphadimidine. Similar finding is stated by Brogden et al. (2003) who found that β-lactams, tetracyclines, aminoglycosides, quinolones, macrolides and sulfonamides are used on commercial cattle dairy farms. The prevalence of AR in commercial bulk samples was 18 %. The finding was agreed by (Khaskheli et al., 2008; Rybinska et al., 1994). The present findings showed higher values in comparison to that of Kang’ethe et al. (2005) who conducted the investigation in Kenya. The variations might be due to the regional variation and the differences in intensity of use of antibiotic drugs in dairy cows.
The results of the current study revealed that the prevalence of AR was positively correlated with the size of the farms. There was significant variation in the prevalence of AR among the small, medium and large dairy farms. As far the authors aware, there are a few studies reported an association between the size of the farms and prevalence of AR during the study. It is assumed this association is due to the higher frequency of antibiotic use in large dairy farms (Chowdhury et al., 2015).
The present study revealed that the milk from farms with sick cows had the highest prevalence of AR than milk from farms with no sick cows. The prevalence of AR in the milk of farms that had cows undergoing treatment was significantly higher than other farms. This might be associated with predominant antibiotic treatments in lactating cows (Mohsenzadeh and Bahranipour, 2008; Chowdhury et al., 2015). As far we know, there are few studies available to discuss the risk factors associated with the prevalence of AR in milk. The authors suggest when the cow sickness occurs, frequencies of treatment using antibiotic drugs are also higher, and ultimately the prevalence of residues are likely higher.
In this study the average concentrations of amoxicillin residue in raw milk was several times higher than the acceptable Maximum Residue Limit (MRL) of amoxicillin residue (40 µg/l) in livestock products (Passantino and Russo, 2008). The finding was higher than those of previous studies (Ghidini et al., 2002; Chowdhury et al., 2015) those found up to 53.7 µg/l amoxicillin residue in raw milk in Iran and 56.16 µg/l in Bangladesh. The oxytetracycline residue in raw milk was approximately two times higher than the acceptable MRL (100 µg/l) prescribed by some authors (Passantino and Russo, 2008). The oxytetracycline residue concentrations in the present study were also slightly higher than that of Kaya and Filazi (2010). The differences in concentrations of oxytetracycline residue in milk may be due to higher doses of antibiotics used during treatment. The ciprofloxacin residue in raw milk was higher than the findings of Chowdhury et al. (2015). But the finding was within the acceptable MRL (147 µg/l) as suggested previously (Passantino and Russo, 2008). The current study revealed that the amoxicillin residue in market milk sample was several times higher than the acceptable MRL as suggested in previous studies (Passantino and Russo, 2008). The concentration of amoxicillin residue in market milk was lower than the raw milk samples. It might be due to the effect of heat during pasteurization. To the best of the author’s knowledge, there are very few literatures available on amoxicillin concentrations in market milk. So, no comparisons can be made with other studies. The result of oxytetracycline concentrations in pasteurized milk in the current study was within the acceptable MRL as reported by previous findings (Passantino and Russo, 2008). The finding of the present study also coincides with the previous study (Abbasi et al., 2011) that found 87.3 µg/l oxytetracycline residue in pasteurized milk in Iran.
CONCLUSION
The overall prevalence of AR was 18% in milk samples of commercial farms. Size of the farms, presence of sick cows and ongoing treatment of cows were identified as significant risk factors for the presence of AR in milk. Boiling reduced the prevalence of AR in milk for amoxicillin, gentamicin, ciprofloxacin and oxytetracycline. The concentrations of amoxicillin and oxytetracycline residue in milk both before and after boiling were higher than the acceptable Maximum Residue Limit (MRL). Legislative bodies should work towards educating farmers and regulating withdrawal periods of antibiotics at the farm level. The present study will contribute towards understanding the level of AR in milk along with the effect of boiling on their concentrations to build public awareness regarding the impacts of AR in human health.
ACKNOWLEDGMENTS
We are very grateful to the authority of Poultry Research and Training Centre (PRTC) laboratory, CVASU who allowed us to test the samples. We are also grateful to the dairy farmers of Chittagong Metropolitan Area (CMA) and Patiya upazila, Chittagong for their support and co-operation. In addition, we would like to acknowledge University Grants Commission (UGC) of Bangladesh for funding the research (UGC/CVASU/RE/2014-2015/23).
Authors Contribution
All the authors contributed significantly to this manuscript. Md Saiful Bari and AKM Humayun Kober designed the experiment, collected and analyzed the data. Md Saiful Bari drafted the manuscript. Goutam Kumar Debnath, Md Ahasanul Hoque and Gouranga Ch. Chanda assisted in data collection and experimentation. All authors revised the manuscript and approved.
CONFLICT OF INTEREST
The authors have dclared no conflict of interest.





