Advances in Animal and Veterinary Sciences
Review Article
Equine Herpesvirus-1 Infection, Clinical Features, Treatment and Control
Emad Beshir Ata1, Alaa Abdelmoneam Ghazy1, Raafat Mohamed Shaapan2*
1Department of Parasitology and Animal Diseases, Veterinary Research Division, National Research Centre, 33 Bohouth St., Dokki, Giza, P.O. 12622, Egypt; 2Department of Zoonotic Diseases, Veterinary Research Division, National Research Centre, 33 Bohouth St., Dokki, Giza, P.O. 12622, Egypt.
Abstract | Equine herpesvirus type 1 (EHV-1) is a worldwide threaten affects the equine industry. The clinical features of EHV-1 infection included the respiratory, abortion, neonatal disease, and neurological forms with a frequently fatal outcome especially in the old age cases. The respiratory form characterized by fever, anorexia and nasal discharges. The abortion could occur in the last third of pregnancy either sporadically or progress into a storm. While in the case of myelencephalopathy, the signs ranged from mild ataxia to severe neurological deficits. Treatment of such cases depends on decreasing the inflammatory signs through using symptomatic and supportive treatment. So, a combination of free-radical scavengers, anticoagulants, and anti-inflammatory drugs, specific anti-herpesvirus drugs (e.g. acyclovir and valacyclovir) especially with the injection route rather the oral one are recommended. Because of the ubiquitous nature of the EHV-1 and the establishment of lifelong latency, elimination of the pathogen from the equine population is difficult. EHV-1 infection results in short-lived immunity which does not prevent re-infection. Although the modified live virus (MLV) and inactivated vaccines are available, it was shown to suppress EHV-1 disease not to limit the viral load. The MLV vaccines have an excellent safety record and can protect horses against clinical disease; however, their efficacy in preventing viremia, abortion, and neurological disease is unclear. Early diagnosis, prevention of further spread and management of clinical cases are the major priorities to control an outbreak. Prevention of virus spreading can be relatively achieved through sound biosecurity measures. This entails quarantine and isolation of new additions for at least a month, cleaning and disinfection of transportation equipment, fomites, and the areas contaminated using disinfectants like chlorine, the quaternary ammonium compounds and the sodium linear alkylbenzene sulfonate but factors like ambient temperature, contamination by organic materials, time of exposure and disinfectant concentration should be considered.
Keywords | EHV-1, Clinical signs, Treatment, Vaccination, Disinfection
Received | December 14, 2019; Accepted | May 11, 2020; Published | June 02, 2020
*Correspondence | Raafat Mohamed Shaapan, Department of Zoonotic Diseases, Veterinary Research Division, National Research Centre, 33 Bohouth St., Dokki, Giza, P.O. 12622, Egypt; Email: rmshaapan2005@yahoo.com
Citation | Ata EB, Ghazy AA, Shaapan RM (2020). Equine herpesvirus-1 infection, clinical features, treatment and control. Adv. Anim. Vet. Sci. 8(6): 668-679.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.6.668.679
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ata et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Equine herpesvirus type-1 (EHV-1) is Equine herpesvirus type-1 (EHV-1) is belong to the family Herpesviridae, subfamily alphaherpesvirinae, genus Varicellovirus, and species Equid alphaherpesvirus 1. It is a ubiquitous and highly contagious pathogen that causes a range of disease severities with outbreaks of notable economic impact (Ata et al.,2 018a, b; Tallmadge et al., 2018). It is a serious worldwide threaten to the horse industry (Lunn et al., 2009). The output of this disease includes one or more of the following clinical signs; severe respiratory manifestations, abortion storm in mares, neurological signs or even death (Walter et al., 2013; Damiani et al., 2014; Ata et al., 2018b). Inhalation of the virus infectious particle to the respiratory tract is the main route of infection, the initial replication of the virus occurs at the upper respiratory tract results in virus shedding in the nasal discharge (Kydd et al., 1994). Fever and respiratory clinical signs may appear, although some horses express subclinical shedding (Burgess et al., 2012). Within the first two weeks of infection, the clinical respiratory signs usually cease but returning of the lymph nodes to the normal size may take more time (Slater, 2007).
Treatment of the infected cases focuses on reducing the inflammation associated with EHV-1-induced vasculitis (Pusterla et al., 2009); by using of symptomatic and supportive care and maintenance of hydration and nutrition (Goehring et al., 2008; Goehring, 2015). The use of specific anti-herpesvirus drugs has also been reported (Henninger et al., 2007).
Both of the modified live virus (MLV) and inactivated vaccines are currently available for protection against EHV-1 (Kydd et al., 2006). Recently, protection against neurological disease under experimental conditions and abortion under field conditions using the RacH-based vaccines was reported (Goodman et al., 2006; Bresgen et al., 2012). To date, there is no clear guideline about emergency vaccination against EHV-1 and 4, which remains a controversial subject (Paillot et al., 2017). Current vaccines offer limited protection from infection or reactivation of the virus. Vaccines presented on the market have been shown to suppress EHV-1 disease and shedding, but may not limit viral load (Goehring et al., 2010a; Bresgen et al., 2012).
Equine herpes viruses are liable or sensitive to many disinfectants. A 1:10 dilution of bleach in water is effective. Both alcohol and bleach disinfectants are inactivated by organic matter. Therefore, all areas must be thoroughly cleaned using soaps or detergents before applying a disinfectant otherwise it is better to use a disinfectant that retains activity in the presence of organic matter such as Phenolics, and accelerated hydrogen peroxide products (CDFA, 2015).
Therefore, the objective of the current review article was to throw light on the most common clinical signs of EHV-1 infection, treatment, and control including the different vaccine types and disinfectants.
Clinical features
Clinical Features was classified into Respiratory, Abortion, neonatal and neurological form of signs (Figure 1).
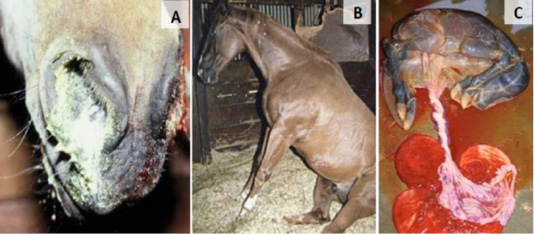
Figure 1: EHV-1 clinical signs; respiratory symptoms (A), neurological features (B) and abortion (C).
Respiratory form
The incubation period observed for natural infections was longer up to 10 days, the respiratory clinical signs that are associated with EHV-1 may include watery nasal discharge, swollen lymph nodes, coughing and loss of appetite and resemble the ‘colds’ affecting horses due to a range of viruses including other types of equine herpes virus. (Slater, 2007) than that observed under experimental settings 1–3 days (Gibson et al., 1992a, b), this suggests that the severe respiratory disease observed in outbreaks may reflect secondary bacterial infections, with mild or asymptomatic primary viral infections acts as a predisposing factor. Infection of native horses with virulent strains of EHV-1 results in an upper respiratory disease characterized firstly by fever, depression, anorexia and progressive lymphadenopathy, as well as ocular and nasal discharges that often progress from serous to mucopurulent (Gibson et al., 1992a), while a cough is not a common clinical feature of EHV-1 infection. An initial leukopenia followed by leukocytosis develop approximately one week following EHV-1 infection coincide with the second peak of fever, and often with the change in the character of nasal discharge (Gibson et al., 1992a). The clinical signs of the upper respiratory disease usually subside within approximately two weeks of EHV-1 infection, although it may take longer for the lymph nodes to return to normal size (Slater, 2007). Severe rhinitis and bronchopneumonia described in association with the natural EHV-1 infections could rarely be reproduced experimentally (Allen and Bryans, 1986; McCulloch et al., 1993). Resolution of such signs usually occurs within 12 days post-infection (Gibson et al., 1992a). The severity of the disease varies with the age of the horse and the level of pre-existing immunity due to natural exposure and/or vaccination (Timoney, 2006). Horses affected by respiratory disease usually recover uneventfully, although the time to recovery may be affected by the presence of concurrent or secondary infections. Some horses may show prolonged poor performance after apparent recovery from EHV-1 respiratory disease (Slater, 2007). It was recorded that, any stressors should be considered in the management of zoological collections to reduce the risk of fatal EHV infections in new hosts (Seeber et al., 2018).
Abortion form
Equine herpes virus-1 is considered as one of the common infectious causes of abortions in mares (Smith et al., 2003). It was the main cause of 8.9 % of the abortion cases in German thoroughbred breeding (Weber et al., 2018).
Abortions due to EHV-1infection usually occur in the third trimester between the 8th and 11th months of gestation, and occasionally may be progressed into abortion storms with a lateral transmission of the virus between horses (Mumford et al., 1994; Patel and Heldens, 2005). Some resistance to abortion could happen if the infection occurred early in pregnancy; this point of view was thought to be related to the reduced number of vascular lesions and viral antigen expression in the endothelial cells of early pregnant mares compared with mares infected late in pregnancy (Smith et al., 1996).
The aborted fetuses are typically fresh and may or may not show marked histopathological changes suggestive for EHV-1 infection. Most abortions occur as sporadic cases and are presumed to have resulted from re-activation of the latent virus, rather than from de-novo EHV-1 infection (Hartley and Dixon, 1979; Laugier et al., 2011).
Previous report showed that, the aborted fetuses due to EHV-1 infection were of normal weight and size (Platt, 1978). Recent study reported that EHV-1 cause large size of fetuses than normal occurs at 201–360 days of pregnancy and in the case of fetuses affected by other factors (Murase et al., 2017).
The aborted fetuses may have jaundice, yellow fluid collections in the thorax and abdomen, pulmonary edema and multifocal Pinhead-sized necrosis foci in the liver with rare presence of fibrin intercalations in the trachea and bronchi (von Oppen and Bartmann, 2001). Although in a severe outbreak in a Germany farm, all aborted fetuses were fresh and enclosed within the fetal membranes. Neither placenta nor gross fetus lesions were detected (Damiani et al., 2014).
Neonatal disease form
Mare’s abortion due to EHV-1 infection could be associated with stillbirth, neonatal foal death (Matczuk et al., 2018).
Some mares exposed to EHV-1 very late in gestation might not abort but instead give birth to a congenitally infected foal that is usually ill at birth (Dixon et al.,1978; Allen and Bryans, 1986), also may be born dead or alive but weak, depressed, polypnoeic and febrile and die within hours or days (Hartley and Dixon, 1979). Other foals may be healthy at birth but succumb to the effects of EHV-1-induced tissue damage within the first week of life (Bryans et al., 1977).
Some foals were normal at birth but later they suffered from fever for 1 week with an increased labored breath and heart rate with normal lung sound. Mucous membranes appeared congested or icteric. The radiography revealed severe, diffuse abnormal interstitial and alveolar densities, increased fluid in the intestinal lumen and the appearance of increased small intestinal wall thickness (Murray et al., 1998).
During an EHV-1 outbreak in a large stud farm, the infected foals showed uveitis and nasal discharge, although pneumonia and colic with intussusception were also detected at the postmortum examination with significant antibody responses in the adult horses, but not in the foals (McCartan et al., 1995).
The affected foals typically die within the first few days of life; while those that survive longer develop progressive interstitial pneumonia that is complicated by secondary bacterial infections and die by approximately 2 weeks of age (Timoney, 2006; Slater, 2007).
Neurological form
Neurological disease due to EHV-1 infection is often referred to as equine herpesvirus myeloencephalopathy (EHM) that may occur as a single sporadic cases or as an outbreak, which is likely to represent endogenous (reactivation of a latent virus) and exogenous (lateral spread of EHV-1) sources of infection, respectively causing devastating losses and has a severe impact on the equine industry (Henninger et al., 2007). This form has become more prevalent in recent years (Gryspeerdt et al., 2010; Barbić et al., 2012; Walter et al., 2013).
Infection of the spinal cord is characterised by multifocal regions of virally infected vascular endothelium, associated with vasculitis, thrombosis and haemorrhage that result in ischaemia and organ dysfunction (Wilson et al., 2019).
The incubation period for EHM is hence difficult to define because the primary EHV-1 infection might have occurred months before the reactivation event that led to the development of neurological disease. Fever has been listed as one of the most consistent clinical signs of EHV-1 infection in several outbreaks of EHM. In these outbreaks, the interval between the first detection of fever and the development of neurological signs typically ranged from 4–9 days. Neurological deficits appeared after cessations of viremia, typically 1–4 days after resolution of the second febrile period in the biphasic temperature profile showed by EHV-1 infection (Henninger et al., 2007; Walter et al., 2013).
Some EHV-1 isolates appear to be more likely to induce EHM than others (Nugent et al., 2006; Goodman et al., 2007), although all EHV-1 should be considered to be potentially neuropathogenic (Lunn et al., 2009; Pronost et al., 2010). Experimentally, however, it is difficult to consistently reproduce severe neurological disease similar to that usually observed in field outbreaks (Goehring et al., 2010b). This suggests that factors other than the genetic make-up of the virus are also important for the development of EHM following EHV-1 infection.
Clinical signs of EHM range from mild ataxia to severe neurological deficits in a recumbent horse. Neurological signs appear suddenly and are not usually accompanied by respiratory disease. Weakness in the hind limbs, ataxia, sensory deficits in the perineal area and hind limbs, oedema at the lower abdomen, as well as bladder dysfunction characterized by atony, urine retention, and incontinence were recorded. More severely affected horses cannot support their weight, due to paresis, complete paralysis of hind limbs, or even tetraplegia. The above mentioned clinical signs of EHM have been recorded in both experimental (Allen, 2008; Goehring et al., 2010b) and field infections (Van Maanen et al., 2001; Henninger et al., 2007, Negussie et al., 2017). It looks that, EHM in donkeys could be more severe and or even fatal without clinical signs of paralysis, and recumbency was frequently observed especially in the old female infected animals (Negussie et al., 2017).
Treatment
The treatment regimens for such cases are using symptomatic treatment and nursing. Mainly, supportive care in cases of recumbency, maintenance of hydration and nutrition for maintaining the nutritional needs and frequent bladder and rectal evacuation and reducing the distress of the affected horse (Olsen, 2001; Goehring et al., 2008; Pusterla et al., 2009; Goehring, 2015).
Treatment focuses on reducing the inflammation associated with EHV-1 induced vasculitis (Pusterla et al., 2009). Most veterinarians used a combination of free-radical scavengers (e.g. dimethyl sulphoxide), anti-inflammatory drugs (e.g. flunixin meglumine, dexamethasone, prednisolone) although their capacity to affect against the development of the lesions of EHM is unknown, the use of acetylsalicylic acid (aspirin) in suspected EHM cases at the time of fever, and vitamin E supplementation for its presumed beneficial effect of free radical scavenging within the CNS, has been proposed by Goehring et al. (2005). In horses that require urinary catheterisation, the administration of antimicrobials (e.g. trimethoprim-sulfamethoxazole, ceftiofur) is recommended in order to reduce the risk of bacterial cystitis (Pusterla et al., 2009).
It was reported that, EHV-1 activates platelets through virus-associated tissue factor-initiated thrombin generation that participate in thrombus formation by providing a surface to localize coagulation factor complexes. Accordingly, using of anticoagulants that suppress thrombin generation or platelet inhibitors that impede post-receptor thrombin signaling (phosphodiesterase antagonists) would inhibit EHV-1-induced platelet activation (Stokol et al., 2016). Interestingly, subcutaneous injection of 25000 IU heparin to horses during EHV-1 outbreak resulted in lower EHM incidence (3.2%) than untreated horses (23.3%) (Walter et al., 2016). So, it could be used as adjunctive therapy to reduce the EHV-1serious complications.
The proinflammatory mediator‐induced upregulation of adhesion molecules on cell surfaces is a proposed mechanism that is likely involved in the pathogenesis of endothelial cells infection and EHM (Goehring et al., 2017). Corticosteroids are immunosuppressive drugs and could aid in the control or prevention of the cellular response adjacent to infection of CNS endothelial cells (Goehring et al., 2008). Furthermore, the anti-inflammatory medications reduce the infection of endothelial cells may be through decreasing contact between EHV-1 infected peripheral blood mononuclear cells (PBMC) and endothelial cells (Goehring et al., 2017). Thereby potentially reducing vasculitis, thrombosis and the resultant neural injury.
The use of specific anti-herpesvirus drugs has also been reported. Although, it is difficult to assess because of the many confounding factors typically present in an outbreak situation. The mechanism of action of acyclovir (9‐[2‐(hydroxyethoxy) methyl] ‐9H‐guanine) and of related antiviral compounds including ganciclovir and penciclovir, is inhibition of DNA synthesis in herpesviruses (Hayden, 2001).
Administration of acyclovir appeared to be associated with decreased severity of disease and increased surviving (Henninger et al., 2007). Acyclovir and ganciclovir are the first-line antiviral compounds for the treatment of herpes simplex viruses and human cytomegalovirus diseases, respectively (De Clercq, 2013; De Clercq and Li, 2016), the in vitro efficacy against different equine herpesviruses was proved (Thieulent et al., 2019). However, other researchers have reported poor bioavailability of acyclovir in the horse and variable serum time profiles of the drug (Wilkins et al., 2005).
Assessment the anti-EHV1 activity of-5021 [(1′S,2′R)-9-[[1′,2′-bis(hydroxymethyl)cycloprop-1′-yl]methyl]guanine] in equine embryonic lung cells and equine nasal explants proved that it is 15 times potent than the acyclovir in inhibition of the virus replication and could be used for the treatment and/or prophylaxis of infections with this virus (Glorieux et al., 2012).
Another nucleoside analogue, valacyclovir, has recently been shown to have better pharmacokinetics in the horse than acyclovir and may provide an antiviral treatment option for EHM affected horses in the future (Garre et al., 2009). Recent in vivo study on equine model revealed the potency of valacyclovir administration in diminishing the fever, shedding of virus and viremia adding to severity but not the risk for ataxia of the experimentally infected animals. The efficacy was high when treatment was started 2 days before viral inoculation. Moreover, during an outbreak of EHM, antiviral treatment could be used at different stages of infection, including horses that have not yet developed signs of viral disease (Maxwell et al., 2017). It is worth to note that, it could be used at a dosage of 40mg/kg body weight, 3 times daily for 5 to7 days for treatment in ponies (Garré et al., 2009). During outbreaks, the oral intake is preferable than the intravenous one, but pharmacokinetic studies cleared that acyclovir given orally to animals is poorly bioavailable with low plasma concentrations (Bentz et al., 2006; Garré et al., 2007). On the other side, the oral administration of valacyclovir reaches concentrations within the sensitivity range of EHV-1 (Garré et al., 2007; Maxwell et al., 2008). Oral administration of famciclovir the prodrug of penciclovir to horses at 20 mg/kg resulted in maximum plasma concentrations occurred between 0.75 and 1.5 hr. (Tsujimura et al., 2010) but penciclovir is very poorly absorbed when administered orally.
The incomplete efficacy of the current specific anti herpes compounds encouraged the scientists to search for alternatives especially plant extracts. Flavonoids are commonly studied due to its variant pharmacological antimicrobial, antiviral, anti-inflammatory, and immunomodulatory protective activities (Fu et al., 2013; Orhan et al., 2010). A promising study to determine the prophylactic and therapeutic effect of quercetin and ethanolic extracts of Bacharis dracunculifolia formulations in an in vivo EHV-1 infection murine model clarified their promising antiviral effect for the treatment of EHV-1 infection (Ferreira et al., 2018).
Immunity and vaccine
Immunity following infection is short-lived and high levels of circulating EHV-1-specific antibodies do not prevent re-infection, suggesting that recognition of the virus by the immune system is compromised (Mumford et al., 1987). Several herpes viruses, including EHV-1, have developed immune evasion strategies (van der Meulen et al., 2006b), including blocking envelope protein expression on cell surfaces. Van der Meulen et al. (2006a) found that 98% of in-vivo infected PBMCs did not express viral envelope proteins on their plasma membrane during viremia.
Vaccination remains the optimal means to prevent infectious diseases in many circumstances; however, there is no evidence that current vaccines can prevent naturally occurring cases of EHM (Henninger et al., 2007). Furthermore, vaccination has been cited as a potential risk factor for the development of EHM, although the supporting evidence is far from conclusive (Ostlund, 1993).
Although Inactivated and modified live vaccines are currently available and attractive tools for prevention of initial infection and spread of the virus via viremic lymphocytes, they are not very effective against EHM (Patel and Heldens, 2005), the immunity after infection and vaccines are typically short-lived and incomplete, thought to be due to the immunomodulatory properties of EHV-1, and our ability to control EHV-1 is limited by our ineffectiveness to elicit protective host immune responses which is a problem shared with a number of other human and veterinary herpesviruses (Allen et al., 2004; Soboll et al., 2014a, b).
An ideal EHV-1 vaccine would not only be safe and lend itself to efficient delivery, it would also invoke strong, persistent local humoral (virus neutralizing antibody) and cellular (cytotoxic T-lymphocyte; CTL) responses at the respiratory mucosal surface in order to block infection and would be required to induce durable systemic humoral and CTL responses to rapidly clear free virus and to destroy virus-infected cells in the event that the mucosal response did not prevent systemic infection (Slater, 2007). Beyond that, it should induce a broad spectrum of responses in young foals to protect them against field challenge that inevitably occurs during their first 2 years and leads to a chronic latent-carrier state (Slater, 2007). Unfortunately, currently available vaccines fall short of these ideals.
While a preliminary experimental challenge study indicated some benefit associated with vaccination with a modified live vaccine (Goodman et al., 2006). No specific recommendation can be made in terms of vaccination for the prevention of EHM at this time. However, based on the presumed similar pathogenic mechanism between EHV-1 abortion and neurologic disease, some likely parallels exist in terms of the requirement for immunological protection (Edington et al., 1986, 1991). The control of cell-associated viremia is thought to be critical for the prevention of EHV-1 abortion and presumably neurological disease. Therefore, the goal of any vaccination program aimed at the prevention of EHV-1 abortion or neurologic disease is to stimulate those immune responses that can reduce or eliminate cell-associated viremia (Kydd et al., 2003).
Of the 17 vaccines containing an EHV-1 antigen marketed in North America or Europe, 15 are inactivated whole virus vaccines and two are MLV. All are administered by IM injection, 15 are licensed to aid the prevention of respiratory disease, and two (all inactivated) are licensed to aid the prevention of abortion (Patel and Heldens, 2005).
Both modified live virus (MLV) and inactivated vaccines are currently available for protection against EHV-1 induced diseases. The MLV vaccines based on EHV- 1 RacH strain are licensed as Rhinomune in the United States and as Prevaccinol in Europe. The MLV vaccines have an excellent safety record and can protect horses against clinical disease; however, their efficacy in preventing viremia, abortion, and neurological disease is unclear (Kydd et al., 2006). Recently, protection against neurological disease under experimental conditions and abortion under field conditions using the RacH-based vaccines was reported (Goodman et al., 2006; Bresgen et al., 2012). Concerning the safety of MLV vaccines, the currently available EHV-1 vaccines are almost safe. The majority of which are combination vaccines with EHV-4 and/or other equine pathogens (Ma et al., 2013).
In general, inactivated vaccines are able to induce high levels of virus neutralizing (VN) antibodies, which, theoretically, play an important role in neutralizing lytically replicating EHV-1. The action of VN antibody should reduce virus shedding in the nasal cavity and dampens clinical symptoms, especially with respect to the respiratory symptoms. Apart from that, humoral immunity is not considered to be the most effective mechanism in protection against abortion or EHM. It has been shown that abortions occur irrespective of high levels of VN antibodies in pregnant mares (Mumford et al., 1994).
In recent studies, the efficacy of inactivated and MLV vaccines was compared. In the study conducted by Goodman et al. (2006), the MLV vaccine (Rhinomune) induced significantly lower VN antibodies than the inactivated vaccine containing inactivated EHV-1 (Fluvac Innovator 6 combination vaccine, Fort Dodge). However, the MLV vaccine reduced virus shedding after challenge to almost undetectable levels. It also prevented neurological signs but only slightly reduced viremia (Goodman et al. (2006)). In another study, the efficacy of the MLV vaccine was compared to an inactivated vaccine with high antigen content for abortion control (Pneumabort K, Pfizer) in an optimized 3-dose vaccination regimen. Clinical signs and nasal shedding after challenge were significantly reduced in both vaccine groups; with a reduction rate greater in case of the MLV group. However, the duration of viremia was only reduced in the inactivated vaccine group (Goehring et al., 2010a). Bresgen et al. (2012) performed another study comparing inactivated and MLV vaccines in pregnant mares and foals on a large stud. Here, a 2-dose application regimen of the MLV vaccine and a 3-dose of EHV-1/EHV-4 inactivated combination vaccine induced a comparable neutralizing antibody response. No significant differences were observed with respect to abortion rates.
It is worthy to note that, only three products [Pneumabort K and Duvaxyn-1, 4 (Zoetis) and Prodigy (MSD-Intervet)] claim to protect against EHV-1-induced abortion. Vaccines that are labeled for prevention of EHV-1-induced neurological diseases are not available (Rosas et al., 2006).
The efficacy of DNA vaccines has also been evaluated. A combination of plasmids expressing gB, gC, and gD or plasmids expressing the IE protein (ICP4) and the early UL5 gene product was used to immunize ponies and were shown to induce only limited immune responses and protection against EHV-1 challenge (Soboll et al., 2006). The failure to induce more complete protection may be due to the fact that the genetic background of animals was not taken into account in these studies. In a later study, ponies expressing a specific MHC class I serological haplotype (A3/B2) were vaccinated with a recombinant modified vaccinia virus Ankara (rMVA) expressing the IE gene. Upon challenge, even though the antibody titres were not increased. Both clinical signs and viremia which is a prerequisite for the prevention of abortion and neurological disease in vaccinated animals were significantly reduced (Soboll et al., 2010).
By deleting virulence-associated genes, a number of EHV-1 mutants have been constructed and tested for vaccine efficacy. An EHV-1 mutant with a deletion in gE and gI was found to be safe for horses but provided only partial protection against EHV-1 challenge (Matsumura et al., 1998). Similar findings were observed in another study, where a gE-negative EHV-1 mutant did not cause any signs of respiratory disease in foals vaccinated intranasally or intramuscularly and reduced virus loads in nasal secretions and viremia after challenge (Tsujimura et al., 2009). EHV-1 mutants devoid of gB or gM were apathogenic for mice and provided protection against challenge infection; however, their safety and protective potential are unknown in horses (Neubauer et al., 1997). In a recent study, it was found that the lately expressed ORF68 protein was not essential for EHV-1 Ab4p growth but was crucial for virus penetration and spreading at the cellular level. Therefore, the constructed EHV-1 ORF68 negative mutant could be a prospective candidate for preparation of marker vaccine to differentiate between the naturally infected and the vaccinated animals (Ata et al., 2018c). Also, studying of the UL11 gene of the Ab4p strain revealed that it is an essential gene for virus growth and could provide a new insights for identifying novel antiviral targets and/or different vaccine design strategies that can be used to improve the current approaches for the control of EHV-1 infection (Badr et al., 2018). Furthermore, evaluation of (EHV-1) gE defective mutant as a MLV in colostrum-deprived Thoroughbred foals resulted in no exhibition of any clinical signs of respiratory disease with remarkable increases in SN antibody titer to EHV-1 after the 2nd inoculation dose. While following a wild type EHV-1 challenge infection, vaccinated foals showed milder clinical symptoms. Additionally, the virus load of nasal shedding and viremia were reduced by vaccination (Tsujimura et al., 2009).
Prevention and control measures
Despite the fact that EHV-1 is a common infection among horses worldwide, the prevention of its associated diseases remains challenging. This partly reflects the complexity of the virus-host interactions and the limited understanding of the factors that are important for the expression of clinically different forms of disease. Prevention and control efforts are also hampered by the fact that many EHV-1 infections occur early in life and are presumably followed by a life-long latency (Dunowska, 2014). Also, currently available vaccines do not confer protection against neurological manifestations of infection (Patel and Heldens, 2005; Slater, 2007). So, understanding these limitations is important for the development of disease control programs.
Because of the ubiquitous nature of EHV-1 and the establishment of lifelong latency, elimination of the pathogen from the equine population is difficult. The current approach to control the EHV-1 infection and disease is based on biosecurity controls and vaccination (Soboll et al., 2014b). The early application of the biosecurity measures not only reduced the effect on the farm but mitigated the potential for the virus to spread to other horse enterprises (McFadden et al., 2016).
These measures can be divided into measures designed to prevent or reduce the likelihood of outbreaks, and measures designed to limit the spread of disease when an outbreak occurs. The impact of abortion outbreaks in broodmare operations has prompted the development of guidelines for the prevention of such outbreaks (Lunn et al., 2009).
The priorities for preparing strategies against an outbreak should support three objectives including early diagnosis, prevention of further spread and management of clinical cases (Lunn et al., 2009). Structuring of serological surveys and assessment of risk factors are required to study and monitor the different epidemiological aspects. The role of early diagnosis is vital and using of specific and sensitive tools like the pecific ELISA for serological diagnosis, PCR and real time RT-PCR for molecular characterization and differentiating between lytic or latent viruses is crucial (Ata et al., 2018a, b). In situations where the preliminary diagnosis means that EHV-1 is suspected, the clinician should proceed with measures designed to contain EHV-1 spread until a specific diagnosis is achieved, or EHV-1 infection is excluded (Allen, 2002).
Prevention of further spread can be relatively achieved through sound biosecurity system in the area of concern, whether it is a farm, racetrack, or horse show. This entails quarantine and isolation of new additions for at least a month, cleaning and disinfection of transportation equipment and fomites and disinfection of any area contaminated by the virus from the aborted fetus and placental membranes (Pusterla et al., 2009).
The biosecurity measures including; movement restriction of horses into, within and from the farm, isolation of the aborted mares in an isolation paddock, and separate clothing and equipment should be used for the different animal groups (Damiani et al., 2014).
The risk of developing EHM was shown to be high in older horses, pregnant mares and mares with foal at foot (Ata et al., 2018b). It is important to have specific rationales to prevent the abortion or neurologic disease in pregnant mares like segregation of pregnant cases from all other horses on the premises. Isolation for a period of not less than weeks of all mares entering the stud farm, including those that are returning after leaving the premises. Subdivision of pregnant mares into small physically separated groups for the duration of gestation is recommended. Stress reduction by avoiding physiological stress, prolonged transport, relocation, poor nutrition, parasitism, and extreme environmental exposure (Allen et al., 2004).
In the case of an EHV-1 outbreak, vaccination can be used in horses at increased risk of exposure. There is some controversy associated with this practice because of the concern that EHM may be associated with a history of frequent vaccination (Henninger et al., 2007). However, there are no reports of vaccination in this circumstance precipitating or exacerbating the occurrence of EHM cases. In previously vaccinated horses, a booster of EHV-1 vaccine can lead to a rapid response and contribute to reduce the spread of the infectious virus (Anon, 2008).
It is important that in case of EHV-1 or EHV-4 infection has been identified, any further fevers in hospitalized horses need to be investigated, and rectal temperatures during a time like this should be collected at least twice daily in all hospitalized horses (Goehring et al., 2010a).
The equine hospital should have alcohol-based hand sanitizer. It is important to have personal protective clothing especially the disposable ones before contacting with animals. Rubber over boots, fabric gowns, and gloves are required to be worn. Grooming tools, thermometer, and stethoscope are assigned to each patient at admission. Additional isolation and barrier precautions are used when managing patients in the isolation building. Dispensers for disposable gloves should be located for hygienic disposal (Goehring et al., 2010c).
Euthanasia should be considered in laterally recumbent horses or sling-supported animals failing to show improvement after a few days or in animals developing severe systemic complications from recumbency as the prognosis for full neurological affected prolonged cases is not generally favourable (Van Maanen et al., 2001).
Disinfection
A high control measures should be applied during any EHV-1 outbreak (Allen, 2002; Slater, 2007). Because it is highly contagious and easily transmitted by direct contact mainly through the infected nasal droplets between infected animals or animals and infected fomites (Ata et al., 2018b). A large amount of the virus particles usually found at the aborted fetus, placenta and the amniotic fluid, therefore appropriate disinfectant should be applied at any contaminated area to minimize the spread of infection (Allen, 2002; Slater, 2007). Multiple EHV-1 outbreaks occurred worldwide although the causative virus could be inactivated easily by the present disinfectants (Allen et al., 2004; Walter et al., 2013; Damiani et al., 2014), which may be due to inappropriate disinfection process that affected by several factors, including ambient temperature or contamination by organic materials, time of exposure and disinfectant concentration (Kahrs, 1995).
The mode of action of disinfectants against viruses has not been fully clarified (Maillard et al., 2013. The free chlorine in the chlorine-based disinfectants may denature viral proteins. Meanwhile, the quaternary ammonium compounds (QACs) disrupt the viral lipid envelope by their surfactant action (Tsujimura et al., 2015).
Interestingly, the sodium linear alkylbenzene sulfonate (LAS) a kind of anionic surfactant foaming agent commonly used in the kitchen but not generally used as a disinfectant. Its virucidal effect on EHV-1, however, was almost equivalent to that of the QACs. Accordingly, routine washing of farm premises like buckets, grooming equipment and clothing with diluted kitchen detergent containing at least 0.05% LAS might be effective in preventing (Allen et al., 2004; Slater, 2007).
QACs, which are relatively less toxic and also odourless and colourless (Kahrs, 1995) when being used at their highest recommended concentrations, it had no virucidal effect on EHV-1 with a 10-min reaction time at 0°C or a 1-min reaction time at room temperature, but it can be used as a disinfectants in case of being diluted with warm water and kept in contact with the objects for a relatively long time (Tsujimura et al., 2015). On the other side, the Chlorine-based disinfectants achieved EHV-1 disinfection in 10 minutes time at −10°C or in room temperature for half a minute, but its activity is reduced in the presence of organic matters, so the disinfectant solution should be replaced periodically (Tsujimura et al., 2015).
Equine herpes viruses are susceptible to many disinfectants. A 1:10 dilution of bleach in water is effective. Both alcohol and bleach disinfectants are inactivated by organic matter. Therefore, all areas must be thoroughly cleaned using soaps or detergents before applying a disinfectant. If an organic material cannot be completely eliminated, it is better to use a disinfectant that retains activity in the presence of organic matter such as Phenolics, and accelerated hydrogen peroxide products (CDFA, 2015).
Conclusion
The EHV-1 infection could have clinical features either one or more of the following respiratory, abortion, neonatal disease, and neurological forms. Although the modified live virus (MLV) and inactivated vaccines are currently available on the market for protection against EHV-1 induced diseases, it was shown to suppress EHV-1 disease not to limit the viral load. Most of the MLV vaccines have an excellent safety record and can protect horses against clinical disease; however, their efficacy in preventing viremia, abortion, and neurological disease is unclear. Prevention of further spread can be relatively achieved through sound biosecurity system in the area of concern. This entails quarantine and isolation of new additions for at least a month, cleaning and disinfection of transportation equipment and fomites using compounds like chlorine, the quaternary ammonium compounds (QACs) and the sodium linear alkylbenzene sulfonate (LAS), but factors like ambient temperature, contamination by organic materials, time of exposure and disinfectant concentration should be taken in consideration.
Authors contribution
The Authors worked cooperatively while collection of information related to this review article.
Conflict of interest
The authors declare that there is no known conflict of interest associated with this publication.
Animal welfare statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to “No ethical approval was required as this is a review article with no original research data”.
References





