Advances in Animal and Veterinary Sciences
Research Article
Protective Effect of Vitamin E against Herbicide Paraquat-induced Enzymatic Leakage and Oxidative Damage in the Liver of Rats
Mostafa A. Shalaby, Shimaa R. Emam, Ahmed M. Soliman*
Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Giza, P.O. Box 12211, Egypt.
Abstract | Paraquat (PQ) is widely used herbicide in various countries. However, PQ intoxication causes liver disease mainly in mammals. To examine the potential ameliorative effect of vitamin E (Vit. E) against PQ induced hepatotoxicity in rats, forty-two rats were distributed randomly into six groups. The first group was kept as control and given corn oil orally. The second group was fed Vit. E (200 mg/kg b.wt.), 3rd and 4th groups were administered 1/4 LD50 of PQ (5 mg/kg b.wt) and 1/2 LD50 of PQ (10 mg/kg b.wt), respectively, while 5th and 6th groups were administered 1/4 LD50 of PQ + Vit. E or 1/2 LD50 of PQ + Vit. E, respectively for 14 days. Blood samples were collected for biochemical analysis and livers were dissected out for biochemical analysis and histopathology. Serum alanine transferase, aspartate aminotransferase and alkaline phosphatase were significantly increased in PQ groups (P=0.003). Serum total protein and albumin were decreased but total bilirubin and lipid peroxidation were increased. The antioxidant body defenses (reduced glutathione, glutathione peroxidase, superoxide dismutase, and catalase) were significantly decreased in PQ groups (P=0.003). PQ induced various histopathological perturbations in the hepatic tissue. Nevertheless, Vit. E administration to PQ-intoxicated rats significantly restored serum and liver tissue biomarkers and ameliorated histopathological lesions in liver tissue. Taken together, Vit. E could be a candidate hepatoprotective agent against liver damage induced by PQ herbicide.
Keywords | Paraquat, Vitamin E, Hepatotoxicity, Antioxidant, Lipid peroxidation, Histopathology
Received | March 22, 2020; Accepted | May 19, 2020; Published | May 28, 2020
*Correspondence | Ahmed M. Soliman, Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Giza, P.O. Box 12211, Egypt; Email: galalpharma@hotmail.com; galalpharma@cu.edu.eg
Citation | Shalaby MA, Emam SR, Soliman AM (2020). Protective effect of vitamin e against herbicide paraquat-induced enzymatic leakage and oxidative damage in the liver of rats. Adv. Anim. Vet. Sci. 8(6): 639-646.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.6.639.646
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Shalaby et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Currently, a strong association exists between environmental pollutants and liver diseases, which provokes great concern to human health (Kim et al., 2019). Paraquat (1,1-dimethyl-4-4-bipiridinium dichloride, PQ) is a non-selective quick-acting contact herbicide that is extensively used in crop cultivation and conservation tillage systems worldwide (Li et al., 2019). Cumulative evidence have shown that PQ is toxic to mammals, where lung toxicity does occur as a significant form of acute intoxication (Dinis-Oliveira et al., 2008; Li et al., 2017). other types of PQ toxicity has also been recorded such as hepatotoxicity (Han et al., 2014; Liu et al., 2018), nephrotoxicity (Kim et al., 2009), neurotoxicity (Rappold et al., 2011), immuno-toxicity (Khilji et al., 2011), and reproductive toxicity (Li et al., 2019). Owing to the high toxicity of PQ together with the functional failure of multiple organs, numerous countries banned PQ use as an herbicide. Nevertheless, owing to low manufacturing cost and convenient application, PQ is widely used in the developing countries with a consequent high intoxication rate even today (Konthonbut et al., 2018; Liu et al., 2018; Zyoud, 2018; Zhang et al., 2018; Khosya and Gothwal, 2012; Wesseling et al., 2001). It is reported that PQ poisoning accounts for up to a third of all suicides worldwide (Sabzghabaee et al., 2010).
The PQ is a dominant free radical producer. Once entered into the cells, PQ is reduced by nicotinamide adenine dinucleotide phosphate (NADPH)-cytochrome P-450 reductase to form PQ mono-cation free radical. The electron conveyed to PQ rapidly moves to oxygen with consequent generation of superoxide anion, and excessive oxidative damage occurs from ROS (Suntres, 2002). The PQ induced hepatic oxidative stress gets evident by a marked increase in lipid peroxidation and a decrease in reduced glutathione (GSH) content. The activity of liver antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) also get decreased (Noriega et al., 2002).
A common underlying mechanism of most hepatotoxicity-inducing pollutants is the formation of reactive free oxygen radicals (ROS) that cause oxidative stress and lipid peroxidation (LPO). A cumulative effect of these events became evident in the form of damage to the membrane of hepatocytes leading to swelling, degeneration, necrosis, and fibrosis of hepatocytes (Selvam et al., 2013; Mehmetçik et al., 2008). During normal physiologic processes, ROS and other free radicals are continuously produced and attack macromolecules, including proteins, lipids, and DNA and cause tissue injury (Halliwell, 1995). It has been widely accepted that oxidative stress plays vital role in the etiology and development of chronic liver diseases (Calabrese et al., 2007; Balmus et al., 2016). Endogenous enzymatic and non-enzymatic antioxidants are necessary for the conversion of ROS to harmless metabolites to safeguard and restore normal cellular functions and metabolism (Bebe and Panemangalore, 2003). Earlier studies showed that antioxidant substances protect cells against the harmful effects of numerous environmental agents (Mohamed et al., 2016; Sarangarajan et al., 2017).
Vitamin E is a fat-soluble vitamin that plays a significant role in sustaining healthy metabolism and physiological functions in the body (Torquato et al., 2020). Owing to its unique antioxidant activity, Vit E regulates oxidation processes in the body (Sepidarkish et al., 2019). Earlier studies reported that Vit. E could normalize the damaging effect of oxidative stress induced by free radicals by preventing cell membranes or proteins damage, or by regulating certain proteins responsible for regulating signal transduction and gene expression (Khallouki et al., 2020). The Vit. E may minimize LPO in biological systems and is considered the first line of defense against LPO guarding cell membranes against the free radicals attack (Zingg, 2007). Based on the biological activity of Vit E, we hypothesized that this natural antioxidant could counteract the PQ induced liver damage. To test this hypothesis, the events of the hepatic enzymes together with the enzymatic and non-enzymatic antioxidants content, were determined. Further to this, the liver architecture was microscopically examined.
Materials and Methods
Tested compounds
The PQ herbicide (C12H14N2, 186.253 g/Mol molecular weight, 175 °C melting point) was used in the liquid commercial form (PQ concentration 200 g/L) (Gramoxone®, Syngenta crop protection company, USA). Vit. E (α-tocopherol) was obtained from Pharco Company for Pharmaceutical, Alexandria, Egypt. It was dispensed in the form of soft gelatin capsules, each containing 1000 mg of alpha-tocopherol and diluted in corn oil.
Chemicals and biochemical kits
Thiobarbituric acid (TBA), trichloroacetic acid (TCA), H2O2 (33%), ethylenediaminetetraacetic acid (EDTA), reduced glutathione (GSH), oxidized glutathione (GSSG), sodium azide, Tris–HCl, 5,50 dithiobis-(2-nitrobenzoic acid (DTNB), b-nicotinamide adenine dinucleotide phosphate (NADPH), potassium dihydrogen phosphate (KH2PO4), sulfuric acid, butanol, and sodium chloride (NaCl) were supplied by Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals were procured from Merck Ltd., SRL Pvt., Ltd., Mumbai, India.
Animal and experimental design
Forty-two mature male Wistar rats (200-210 g b.wt., 8-10 weeks old) were purchased from Laboratory Animals House, Agricultural Research Institute, Giza, Egypt. The rats were kept under hygienic conditions at a room temperature of 22 ºC and 55 % humidity with a 12-hr light / 12-hr dark schedule. The animals were fed standard rat pellets during the experiment period and water was freely provided.
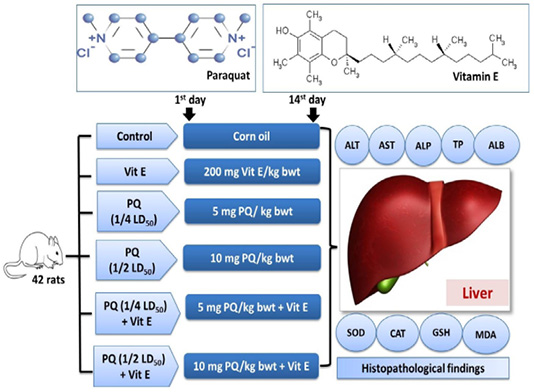
Figure 1: Experimental groups and treatments. Paraquat herbicide (PQ: 1/4 LD50;5 mg/kg b.wt or 1/2 LD50; 10 mg/kg b.wt). Vitamin E (Vit.E; 200 mg/kg b.wt.).
Rats were randomly distributed into six equal groups. The vehicle control group was orally given 2ml corn oil. The Vit E group was orally given 200mg/kg b.wt. Vit. E (Takhshid et al., 2012). The third and fourth group was orally administered 5 mg/kg b.wt (1/4 PQ LD50) and 10 mg/kg b.wt (1/2 PQ LD50), respectively (Afzali and Gholyaf, 2008). The fifth and sixth groups were orally administered 1/4 LD50 of PQ + Vit. E and 1/2 LD50 of PQ + Vit. E, respectively. The experimental design is shown in Figure 1. The animals were dosed for 14 consecutive days. The rats were observed carefully throughout the trial for the signs of toxicity, morbidity, and mortality.
Sampling
At the end of the experiment, the rats were anesthetized, and blood samples were withdrawn by puncture of the tail vein with a small needle. Blood samples were collected in plain tubes, without anti-coagulant to separate clear serum. Blood samples were left to clot and centrifuged at 9000 rpm for 15 min for separating the serum. The separated serum was kept frozen at -20 ºC till serum biochemical analyses. The rats were euthanized by cervical dislocation (Hawkins et al., 2016). Livers were dissected out and washed with physiological saline. The other part of the livers was preserved in 10 % neutral formalin solution and processed for histopathological investigations. The remainder was used for the preparation of tissue homogenate by washing by ice-cooled 0.9% NaCl solution followed by homogenization in ice-cooled 100 ml of 1.5% solution of potassium chloride and 50 mM potassium phosphate buffer solution (pH 7.4) to yield 1% homogenate (W/V). The homogenates were centrifuged at 9000 rpm at 4°C for 15 min, and the supernatants were collected for biochemical analyses. This experiment was carried out according to the guidelines of the Institutional Animal Care and Use Committee, Cairo University, Approval Protocol No.: Cairo University-II-F-59-15 dated December/2018. (Protocol number 2211201809 / 2018).
Serum biochemical analysis
Activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were determined spectrophotometrically (T80 UV/VIS PG instrument Ltd, UK) using commercial test kits (BioMérieux-France) following the protocols of Reitman and Frankel (1957) and Kind and King (1954), respectively. Total protein was determined using Folin phenol reagent by the Lowry method (Lowry et al., 1951). Serum albumin and total bilirubin were chemically determined following the methods of Morgan and Peters (1971) and Brückner (1961), respectively.
Lipid peroxidation and antioxidant assessment in tissue homogenate
Lipid peroxidation was determined by measuring malondialdehyde (MDA), a marker of lipid peroxidation that is formed in terms of thiobarbituric acid reactive substances. Tissue MDA content was determined in liver homogenates according to Ohkawa et al. (1979). Activities of hepatic GPx, SOD, and CAT antioxidant enzymes were determined following the methods of Flohé and Günzler (1984), Nishikimi et al. (1972) and Aebi (1984), respectively. GSH content in liver homogenate was determined colorimetrically using the method of Beutler (1963).
Histopathological examination
The fixed liver`s specimens were trimmed, washed, and dehydrated in ascending grades of alcohol. The samples were sectioned at 5 microns thickness, stained with Hematoxylin and Eosin (H and E), and examined microscopically (Bancroft et al., 2013).
Statistical analysis
Data were presented as means ± standard error of the mean (SE). Statistical analysis between different experimental groups was carried out using a one-way analysis of variance (ANOVA) test followed by Duncan`s multiple range tests [Statistical Program of Social Sciences, (SPSS), Version 17, Chicago, USA], A Probability level of P<0.05 was consider significance.
Results
Effects on liver function indicators
The impact of PQ and Vit. E oral dosing on the liver function parameters is shown in Figures 2 and 3. Serum levels of liver enzymes ALT, AST, and ALP was significantly (P= 0.0034) increased in both doses of PQ herbicide in a dose dependent manner compared to the control group. The TBil content was significantly increased by 46% and 94% in the rats exposed to 5 mg/kg b.wt (1/4 PQ LD50) or 10 mg/kg b.wt (1/2 PQ LD50), respectively compared to the control group. In contrast, Vit. E co-treatment with both PQ levels significantly (P=0.004) depressed the increment in the hepatic enzymes leakage and TBil content compared to its corresponding PQ –exposed groups.
The serum TP and Alb content were significantly (P=0.003) reduced by 51% and 45% in the rats exposed to 5 mg/kg b.wt. (1/4 PQ LD50), and by 77% and 65%, respectively compared to the control group. Contrary to this, the co-administration of Vit. E with PQ significantly restored the TP and Alb content compared to PQ –exposed groups, as demonstrated (Figure 3).
Effects on liver enzymatic antioxidants
As shown in Figure 4, rats were given oral administration of PQ at five and 10mg/kg b.wt. showed a significant decrease (P=0.003) in activities of SOD, CAT, and GPx enzymes in liver tissues compared to the control group. On the other hand, an oral administration of PQ in combination with Vit. E produced a significant increase (P=0.004) in activities of SOD, CAT, and GPx enzymes in liver tissues as compared to those groups that were given oral administration of PQ exclusively.
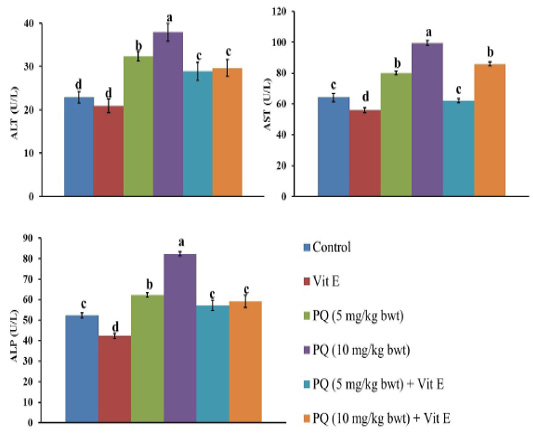
Figure 2: Effect of vitamin E on liver function indicators in administered male Wistar rats.ALT: Alanine transaminase; AST: Aspartate transaminase; ALP: Alkaline phosphatase. Data are expressed as the mean ± SE (n= 7 replicates). Columns carrying different superscripts are significantly different (one-way ANOVA) (p < 0.05).
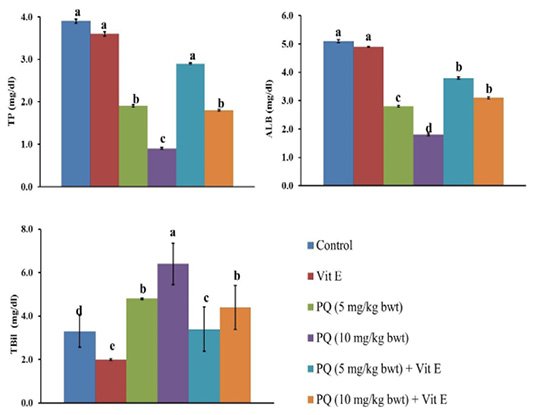
Figure 3: Effect of vitamin E on liver function indicators in paraquat herbicides (PQ) administered male Wistar rats.TP: total protein; ALB: albumin; TBil: total bilirubin. Data are expressed as the mean ± SE (n= 7 replicates). Columns carrying different superscripts are significantly different (one-way ANOVA) (p < 0.05).
Effects on liver non-enzymatic antioxidants
The GSH content in the liver of rats decreased by 26% and 37 % following exposure to 5 and 10mgPQ /kg b.wt., respectively compared to the control group (Figure 5). Nevertheless, Vit. E and PQ co-treatment significantly restored GSH content compared to PQ-treated groups. Moreover, the GSH was normalized in the Vit. E + PQ (5 mg/kg b.wt.) to the extent that no significant difference was detected compared to the control group.
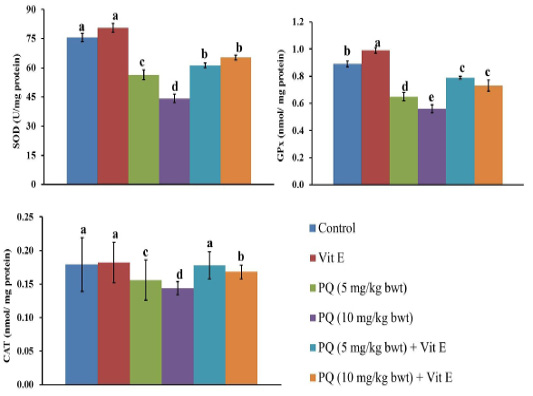
Figure 4: Effect of vitamin E on antioxidant enzymes in paraquat herbicides (PQ) administered male Wistar rats.SOD: superoxide dismutase; GPx: glutathione peroxidase; CAT: catalase. Data are expressed as the mean ± SE (n = 7 replicates). Columns carrying different superscripts are significantly different (one-way ANOVA) (p < 0.05).
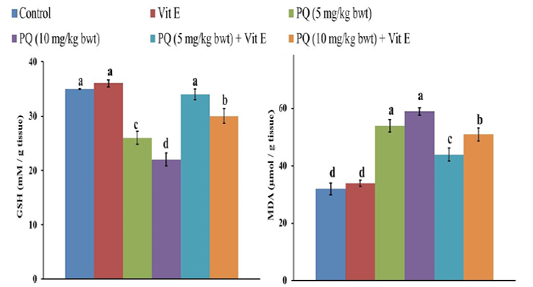
Figure 5: Effect of vitamin E on reduced glutathione (GSH) and malondialdehyde (MDA) content in paraquat herbicides (PQ) administered male Wistar rats. Data are expressed as the mean ± SE (n= 7 replicates). Columns carrying different superscripts are significantly different (one-way ANOVA) (p < 0.05).
Effects on LPO in the liver tissue
There was a significant increase (P= 0.004) of MDA content, the indicator of LPO in the liver, after the administration of 5 and 10 mg PQ /kgb.wt.to rats by 69% and 85%, respectively compared to the control group (Figure 5). Nonetheless, the rats treated with PQ and Vit. E showed a significant decrease (P= 0.003) in MDA content compared to PQ-treated groups.
Histopathological findings
Histopathological examination of the liver of healthy and Vit. E treated groups revealed the normal histological structure of hepatic lobule, which consisted of a central vein surrounded by normal hepatocytes arranged in radiating rows (Figure 6A). The liver of rats intoxicated with PQ herbicide at 5 mg/kg (1/4 LD50) showed moderate congestion and cytoplasmic vacuolation of hepatocytes (Figure 6B). Rats intoxicated with PQ at 10 mg/kg induced an increase in the fibrous connective tissue in the portal area associated with few mononuclear inflammatory cells infiltration and sinusoidal dilatation (Figure 6C). Liver sections of rats that were fed PQ at 10 mg/kg plus Vit. E revealed the almost typical histological architecture of hepatic lobule (Figure 6D).
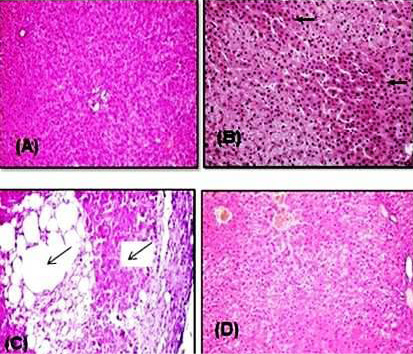
Figure 6: Representative photomicrograph of H&E stained rat liver sections. (A) Control rat showing normal hepatocytes that arranged in radiating rows in between there are normal sinusoids. (B) Rat intoxicated with PQ herbicide at 5 mg/kg (1/4 LD50) showed a moderate congestion and cytoplasmic vacuolation of hepatocytes (arrows). (C) Rat intoxicated with PQ at 10 mg/kg induced an increase in the fibrous connective tissue in the portal area associated with few mononuclear inflammatory cells infiltration and sinusoidal dilatation (arrows). (D) Rats given orally PQ at 10 mg/kg plus Vit. E revealed almost normal histological architecture of hepatic lobule.
Discussion
Despite of the broad international movement for a global ban of PQ use, a very potent weed control activity together with its low price are strong reasons for continuous use in most of the developing countries worldwide (Botta et al., 2020; Wesseling et al., 2001). Therefore, this study aimed to evaluate the ameliorative effect of Vit. E against hepatotoxicity induced by sub-chronic toxicity of PQ herbicide in rats. The main cellular antioxidant enzymes are superoxide dismutase (SOD), glutathione peroxidase and catalase (CAT) and glutathione (GSH), (Abdeen et al., 2019a, 2019b, 2019c; Aboubakr et al., 2019; Aboubakr et al., 2020; Elkomy et al., 2016).
Rats that were fed 5 and 10 mg/kg b.wt. of PQ for two weeks showed a significant increase in serum hepatic enzymes (ALT, AST, and ALP) activities, total protein and albumin but elevated total bilirubin. Liver enzymes are secreted into the blood after a hepatocellular injury, resulting in an increase in their activities in serum samples (Zeinvand-Lorestani et al., 2018). Lipid peroxidation is known to disturb the integrity of cellular membranes, leading to leakage of cytoplasmic enzymes (Selvam et al., 2013; Mehmetçik et al., 2008). Therefore, increased activities of these enzymes in the serum could be due to severe histopathological damages which have been reported previously by other due PQ (Zeinvand-Lorestani et al., 2018; Wesseling et al., 2001). The detailed findings of serum analysis were in agreement with those reported previously in various studies (Chohan et al., 2010; Wershana, 2001; Suntres, 2002; Noriega et al., 2002; Zeinvand-Lorestani et al., 2018; Ray et al., 2007). Moreover, the toxicity of PQ includes an enhanced lipid peroxidation, decreased GSH, inhibited antioxidant enzymes (SOD, GPx, and CAT) in liver homogenates, and induced hepatic histopathological alterations. These findings were similar to those reported previously by others (Noriega et al., 2002; Zeinvand-Lorestani et al., 2018; Matsumori et al., 1984). The liver parenchyma was infiltrated with inflammatory cells, mostly lymphocytes, and macrophages. Fatty degenerative changes were also observed in hepatocytes (Chohan et al., 2010).
The mechanisms of PQ toxicity comprise the superoxide anion release, which consequently leads to the generation of more toxic ROS like hydroxyl radical and hydrogen peroxide. The cellular NADPH oxidation, the critical source of reducing equals for the PQ intracellular reduction, leads to the disruption of essential NADPH-requiring biochemical processes (Suntres, 2002). The PQ induced hepatic oxidative stress can be evident by a marked increase in lipid peroxidation and a decrease in GSH content. The activity of liver antioxidant enzymes, SOD, CAT, and GPx, was also decreased by PQ (Noriega et al., 2002). The involvement of ROS production and enhancement of lipid peroxidation has been implicated in the toxicity of PQ (Ray et al., 2007; Morán et al., 2010). Moreover, PQ induced hepatotoxicity by producing oxidative stress, inflammation, and by modulating xenobiotic-metabolizing, machinery has also been reporeted (Chohan et al., 2010; Noriega et al., 2002; Ahmad et al., 2013). Herein, co-administration of Vit. E with PQ restored the above mentioned parameters in the serum. It is well known that Vit. E regulates all oxidation processes in the body as it acts as a powerful antioxidant. Previous studies showed that intake of Vit. E could normalize the damaging effect of oxidative stress induced by free radicals (Azzi, 2007; Zingg, 2007).
The results of the present study revealed a significant increase in MDA, the indicator of LPO in the liver, after oral administration of 5 and 10 mg/kg of PQ to rats for two weeks. Estimation of lipid peroxidation has been used as a biomarker for oxidative stress in several previous studies (Zeinvand-Lorestani et al., 2018; Wesseling et al., 2001). It can also occur as a consequence of the imbalance between the antioxidant system and the pro-oxidant state generated by insecticide toxicity. The increase in MDA observed in the liver following PQ exposure could probably be ascribed to an excessive production of ROS, which could be related to hepatocytes enzyme leakage.
Conclusion
The biochemical and histopathological endpoints of the present study highlight that natural antioxidants such as Vit. E could efficiently safeguard PQ-induced hepatic injury in rat model via enhanced antioxidant defense system and decreased LPO. Further studies should be done to further as certain study outcomes and molecular mechanisms of these effects.
Acknowledgment
The authors wish to thank Prof. Dr: Adel Baker (Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt) for his comments on the histopathological study.
Authors Contribution
Mostafa A. Shalaby planned this study and revised the manuscript. Ahmed M. Soliman and Shaimaa R. Emam performed the experimental work, samples collection and all laboratory tests. All authors shared manuscript writing, drafted and approved the final manuscript.
Conflict of Interest
The authors have declared no conflict of interest.
References





