Advances in Animal and Veterinary Sciences
Research Article
Enterotoxigenic Profiles of Virulent Bacillus cereus Isolated from Dairy Environments: Antimicrobials Resistant Pattern and Sporicidal Disinfectants Efficacy
Ahmed S. Ahmed1*, Abdullah F. Alsayeqh2, Hassan Mahmoud Diab3
1Department of Food Hygiene and Control (Milk Hygiene), Faculty of Veterinary Medicine, South Valley University, Qena 83523, Egypt; 2Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, 6622, Buraidah 51452, Saudi Arabia; 3Department of Animal and Poultry Health and Environment, Faculty of Veterinary Medicine, South Valley University, Qena 83523, Egypt.
Abstract | The study was aimed to characterize Bacillus cereus using culture-based and PCR techniques among 390 samples including milk, dairy products, water and environmental specimens. Antimicrobial activities of disinfectants and bioactive peptides were performed using European Suspension Test and liquid-broth method. Overall of 29.5 and 67.4% among dairy and environmental samples were positive for B. cereus. The prevalence of B. cereus in household milk, rice milk, water and street vendor milk was 37.5, 36.4, 33.3, and 30%, respectively. Moreover, the prevalence in the rinse water, used bedding material, pasture grass and pasture soil were 38.5, 37.5, 37.5 and 33.3%, respectively. HblA, hblD, hblC were detected in 42.2, 26.7, 24.4% and the nheA, nheB, nheC occurred in 31.1, 33.3, 24.4%, respectively. B. cereus isolates showed resistance to Amoxicillin, Ampicillin, Penicillin, Cephalexin and Tetracycline with 68.9, 62.2, 62.2, 57.8, and 51.1%, respectively. Hyperox and CIDEX disinfectants have extremely rapid bactericidal activity at concentrations 2 and 2.4% after five minutes of exposure times under clean condition resulting in 10.3 and 9.7 log reductions, respectively. Adding organic substances results in a marked decline of the bactericidal activity for both disinfectants. CIDEX exhibited lower Sporicidal efficacy than Hyperox under clean conditions at various exposure times/temperatures. Under the dirty condition, Cidex showed similar or slightly higher sporicidal activity than Hyperox. At 4 hrs/ 20 & 40 ºC under dirty conditions, 4.8, 5.6 and 4.6, 5.2 log reductions for CIDEX and Hyperox, respectively were observed. P-CWP and P-CCP showed effective antimicrobial action resulting in 10 and 8 log reductions at a concentration of 2 mg/ml, respectively against B. cereus. However, CCP and CWP exhibited little to moderate antimicrobial activity. The prevalence of Eneterotoxigenic virulent B. cereus and their drug resistance profiles diversity suggest that the examined dairy environments in villages of Sohag governorates, Egypt poses animal and public health threats.
Keywords | Bacillus cereus; Milk & Milk products; Water; Disinfectants; Antimicrobial biopeptides
Received | October 30, 2019; Accepted | January 17, 2020; Published | May 02, 2020
*Correspondence | Ahmed Shaban Ahmed, Department of Food Hygiene and Control (Milk Hygiene), Faculty of Veterinary Medicine, South Valley University, Qena 83523, Egypt; Email: ahmed_shaaban@vet.svu.edu.eg
Citation | Ahmed AS, Alsayeqh AF, Diab HM (2020). Enterotoxigenic profiles of virulent bacillus cereus isolated from dairy environments: antimicrobials resistant pattern and sporicidal disinfectants efficacy. Adv. Anim. Vet. Sci. 8(5): 543-557.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.543.557
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The genus Bacillus is a Gram-positive, aerobic-to facultative, spore-forming rod-shaped bacterium. It is ubiquitous in nature and constituted the majority of aerobic spore-formers flora that likely to inhabit water-damaged indoor environments and foods (Mohamed et al., 2016). Those thermotolerant spore formers constitute a considerable risk towards keeping quality of both pasteurized and refrigerated dairy products (Caamaño-Antelo et al., 2015; Singh et al., 2015).
There are different species of Bacillus, of which the Bacillus cereus is the most important species since its classification as a common food contaminant involved in both food spoilage Yusuf et al. (2018) as well as food poisoning that usually occurs in two types of illness: the emetic and diarrheal syndromes.
The diarrheal syndrome is due to five enterotoxins have been detected. Hemolysin BL (Hbl) and nonhemolytic enterotoxin (Nhe) have been identified as the major virulence factor among the various enterotoxins of B. cereus (Cui et al., 2016). Hemolysin BL consists of binding protein B, lysing proteins L1, and L2 encoded by hblA, hblC and hblD genes, respectively. While nonhemolytic enterotoxin has three proteins moieties B, L1 and L2 encoded by the three genes nheA, nheB and nheC, respectively (Ombui et al., 2008).
Antibiotic therapy is still the most important treatment method for B. cereus infections. However, antibiotic resistance profiles of B. cereus isolates are of concern for public health (Cui et al., 2016). Acquired resistance to antibiotics is mainly due to antibiotic misusage or acquisition of transferable multidrug resistance genes (Gao et al., 2018). However, there is no report on the antimicrobial susceptibilities of B. cereus isolates from milk, milk products, water and farm environment in Egypt.
Many sporicidal disinfectants, including peroxygens glutaraldehyde, and chlorine-releasing agents (hypochlorite) are commercially available and effective; however, most require long contact times (Russell, 1990). Peracetic acid is a mixture of acetic acid and hydrogen peroxide. It is a very strong oxidizing agent and is considered bactericidal, and sporicidal. It also retains its activity in the presence of organic matter. Peracetic acid is used in food processing and handling as a sanitizer and disinfectant for food contact surfaces (Marquis et al., 1995). Glutaraldehyde was an effective sporicide. CIDEX Activated Dialdehyde Solution is a 2.4% activated glutaraldehyde solution available commercially and has been in use for many years (Lane et al., 1996; Tennen et al., 2000).
Quaternary Ammonium Compounds (QAC) disinfectants are effective against gram-positive and gram-negative bacteria but less or not effective against bacterial spores. Their efficiency is reduced markedly in the presence of organic matter (Anonymous, 2005).
In recent years, the role of milk proteins as the main source of a wide range of biologically active peptides has been acknowledged worldwide (Sindayikengera and Xia, 2005). The antimicrobial activities of milk protein-derived peptides are very diverse, ranging from those with a prebiotic effect, peptides with the ability to prevent the attachment or invasion of pathogen microorganisms, to peptides killing or inhibiting the growth of microorganisms (Hernández-Ledesma et al., 2013). Many peptides with antimicrobial activity have been released from bovine milk proteins, either during fermentation of milk or through enzymatic hydrolysis; these antimicrobial peptides have been found to have activity against many gram-positive and gram-negative bacteria, as reviewed by (Akalın, 2014).
The present study aimed to investigate the prevalence of Bacillus species especially B. cereus in milk, milk products, water and dairy cattle macro-environmental samples in some villages of Sohag, Egypt, in addition to detection the hbl and nhe toxin genes in B. cereus isolates by polymerase chain reaction (PCR). The antibiotic susceptibility profiles of the isolated strains for 10 antimicrobial agents were assessed. The antimicrobial activity of disinfectants and milk proteins hydrolysates against isolated and identified B. cereus strains will be evaluated in vitro. Innovative approaches are necessary to address the health needs of those targeted villages communities. The findings and recommendations of the current study will notify relevant health decision-makers to take the necessary counteract measures.
Materials and methods
Experimental Design
Rationale of the study: The current study was designed to target the household rearing of cattle in Sohag governorate, Egypt to determine the prevalence and monitor the main source of Bacillus species especially B. cereus in cow’s house environment and local dairy shops. An initial survey was conducted to establish the bacteriological status of milk (household and street vendor milk), milk products (Baladi yoghurt, Kareish cheese, Butter and rice milk), water (drinking and rinse water) and environmental samples (bedding materials, pasture grass, pasture soil and air) with optimum treatment using milk proteins hydrolysates and some disinfectants against the recovered virulent B. cereus strains.
The surveyed localities do not rely on supplementary feeding or routine antibiotics use. There was also no record of B. cereus infection among these localities. Economic, environmental and societal factors are coexisted in those selected villages. The Economic aspects is leading factor resulted in rearing of dairy cattle inside the farmers house to use their raw milk as source of income. Moreover, lacking of the basic requirements for housing of dairy cattle and primitive sanitary measures including selling of raw milk without any heat treatment created threats to animal and public health. B. cereus infections remain a major public health issue in those selected sites to which the public health concept principles represent a promising pathway.
Collection of Samples
A total of 390 samples out of milk and milk products (180 samples), water (30 samples), environmental samples (150 samples) and air (30 samples) were collected from dairy cattle rural house and local dairy vendors.
From local dairy houses and vendors, a total of 60 raw milk samples (household and street vendor milk) were collected in clean sterilized 50 ml Falcon™ conical tubes. Household and street vendor milk are considered the most important raw milk samples supplied to the public in Sohag governorate, Egypt. Additionally, 30 milk product samples were also collected from each of the following categories, including; Baladi yoghurt, Kareish cheese, Butter and rice milk. All samples were transported to the laboratory and were stored at -20 ºC until further analysis.
Sixty drinking and rinse water samples were collected out of local water sources inside framer houses in clean sterile transparent 500 ml capacity glass bottles fitted tightly with ground glass stoppers. The samples were immediately placed in a lightproof insulated box containing melting ice or ice-packs with water to ensure rapid cooling. The box used to carry samples was cleaned and disinfected after each use to avoid contaminating the surfaces of the bottles and the sampler’s hands (WHO, 1971).
A total of 60 bedding materials (new & used) and 30 (pasture grass) were collected according to Rendos et al. (1975). The total amount of material (approximately 250 g) collected in sterile plastic bags. Ten grams were chopped into fine particles with sterile scissors then added to 90 ml of sterile 0.1% peptone solution in a sterile glass bottle. The prepared bottles were then mixed thoroughly by manual agitation for 5 min. The mixture then aseptically strained through sterile gauze; the filtrate was collected in a sterile flask for further analysis.
A total of thirty pasture soil samples were collected according to Clegg et al. (1983). Each sample was removed at a depth of 5 cm from different places of the house and transferred to a sterile glass bottle, fitted with a sterile ground glass stopper. After thorough mixing of each soil sample, one gram was crushed well in a sterile mortar with 99 ml of the sterile normal saline solution then aseptically strained through sterile gauze; the filtrate was collected in a sterile flask for further analysis.
Thirty air samples were collected from the examined animal enclosures by liquid impinger air sampler according to Cown et al. (1957). About 50 ml of sterile saline solution was used for collecting the airborne bacteria. The liquid impinger was adjusted at a rate of 5 liters/min. During sampling, the liquid impinger was moved inside the animal house to trap all suspended dust particles. The collected samples were carried in the icebox to the laboratory for bacteriological examination.
Isolation and Identification of the Bacillus Species
The isolation of Bacillus species is adopted according to (Harrigan, 1998; Banykó and Vyletělová, 2009). About 25 ml/g of each sample was aseptically homogenized in 225 ml of nutrient broth for primary enrichment and incubated at 34 ºC for 24 h. Then cultures were streaked on the Mannitol egg yolk-polymyxin (MYP) agar plate supplemented with egg yolk, mannitol, and Polymyxin B and incubated at 34 ºC for 24-48 h. Biochemical identification according to Varadaraj (1993); Banykó and Vyletělová (2009); Bottone (2010).
Detection of B. cereus Virulence Genes
The PCR-technique was applied for the detection of six virulence genes using six sets of primers. Those genes were hemolysin BL (HBL) hblA, hblC, hblD and nonhemolytic enterotoxin (NHE) nheA, nheB, nheC genes. Primer sequences and amplicon size used in this study were presented in Table 1 as described by Yang et al. (2005) and Ombui et al. (2008). PCR was applied following QIA amp DNA mini kit instructions (Catalogue no. 51304); Dream Taq Green PCR Master Mix (2X) (Thermo Scientific) Cat No. K1081 (Sambrook et al., 1989). Positive PCR products for each gene were detected and visualized in single 1.5% agarose gel electrophoreses stained with ethidium bromide, viewed by U.V. light.
Antibiogram Pattern of B. cereus
All B. cereus isolates were examined for their antibiogram pattern by disc diffusion technique as described by Bauer et al. (1966) against a panel of 10 antibiotics. The selection of antibiotics (10) was based on their common use in human and animals in Egypt which included amoxycillin (20 µg), ampicillin (10 µg), cephalexin (30 µg), chloramphenicol (30 µg), ciprofloxacin (10 µg), erythromycin (15 µg), gentamicin (10 µg), penicillin (10 unit), streptomycin (10 µg), tetracycline (30 µg). All antibiotics used were obtained from Oxoid, UK. The results were interpreted as sensitive or resistant based on CLSI interpretive standards (2007).
Antimicrobial Activity of Disinfectants and Milk Proteins Hydrolysates against B. cereus
Microorganisms: Recovered B. cereus virulent strains were enriched in tryptic soy broth before cultivation on MYP agar for 24 hrs at 37 °C before assay.
Disinfectants: The study aimed to evaluate the bactericidal and sporicidal activity of four disinfectants on B. cereus and its spores: CIDEX® is an activated dialdehyde solution,
Table 1: PCR protocol including primer sequences, Amplicon size and amplification reactions a.
| Target gene | Primers sequences | Amplified segment (bp) |
| hblA | ATTAATACAGGGGATGGAGAAACTT |
237 |
| TGATCCTAATACTTCTTCTAGACGCTT | ||
| hblC | CCTATCAATACTCTCGCAACACCAAT |
386 |
| TTTTCTTGATTCGTCATAGCCATTTCT | ||
| hblD | AGATGCTACAAGACTTCAAAGGGAAACTAT |
436 |
| TGATTAGCACGATCTGCTTTCATACTT | ||
| nheA | ATTACAGGGTTATTGGTTACAGCAGT |
475 |
| AATCTTGCTCCATACTCTCTTGGATGCT | ||
| nheB | GTGCAGCAGCTGTAGGCGGT |
328 |
| ATGTTTTTCCAGCTATCTTTCGCAAT | ||
| nheC | GCGGATATTGTAAAGAATCAAAATGAGGT |
557 |
| TTTCCAGCTATCTTTCGCTGTATGTAAAT |
a Primer design and amplification process steps as published by yang et al., 2005 and Ombui et al., 2008.
2.4% glutaraldehyde (Johnson & Johnson Medical Egypt, Cairo, Egypt), Hyperox®, is a colorless aqueous formulation of peracetic acid, hydrogen peroxide, acetic acid and surfactant (Antec International Limited, United Kingdom), Evans Hypochlorite contains a blend of sodium hypochlorite solution (Evans Vanodine International plc, United Kingdom) and BioSentry® 904, quaternary ammonium compounds base (HACCO, Inc., USA). All disinfectants were handled according to the manufacturer’s instructions.
Evaluation of the bactericidal activity of disinfectant: The test method is based on the suspension test according to European Norms (EN) 1276 (BS EN 1276:1997) which specifies a quantitative suspension test for the evaluation of the bactericidal activity of chemical disinfectant used in the food industry. The bacterial suspension was prepared from fresh B. cereus strains isolated during the current study. The suspension was used to simulate clean condition without interfering substance and dirty dairy environment condition through adding milk 4% to form a bacterial-interfering substance mixture. One and five minutes exposure times were chosen. The log count of B. cereus was determined after plate counting on the Mannitol egg yolk-polymyxin (MYP) agar plate.
Evaluation of Sporicidal activity of disinfectant: For the preparation of spore suspensions, wash the growth from a fresh B. cereus culture with sterile physiological saline onto the surface of a Roux bottle containing 300 ml of AK Agar #2 (Sporulating Agar). Incubate the bottles for 5 days at 35 ± 2 ºC and wash off the resulting growth into 50 ml of sterile physiological saline. Centrifuge the suspension and decant and discard the supernatant fluid. Resuspend the sediment in sterile saline and heat shock the suspension at 70 ºC for 30 minutes (Wehr and Frank, 2004). To represent the in-use situation in the dairy industry concerning exposure time, temperature and interfering substances, tests were carried out in the presence of milk (4% v/v) as an organic interfering substance (Te Giffel et al., 1995).
The disinfectant quantitative suspension test for the evaluation of sporicidal activity was performed using the approach outlined in (BS EN 13704:2002) and according to European Norms (EN) suspension sporicidal tests (BS EN 14885:2006). Spores in suspension with/without interfering substance were exposed to disinfectants for specified exposure times: 0.5, 1, 2, 3, and 4 hrs at 20 °C and 40 °C; respectively. The neutralizers used in this study were according (Russell, 1981; Espigares et al., 2003).
Hydrolysis of cow milk proteins with pepsin enzyme:
Separation of caseins and whey proteins were performed according to (Ahmed et al., 2015); briefly fat was removed from 250 ml raw milk by centrifugation and then the skimmed milk was passed through three layers of gauze. The defatted milk was adjusted to pH 4.6 with 10% acetic acid, followed by centrifugation. The fractions cow casein proteins (CCP) and cow whey proteins (CWP) were lyophilized.
In vitro pepsin digestion of CCP ad CWP: The CCP and CWP were dissolved in Milli-Q water and adjusted to pH 3.0 with HCl. Pepsin in 1 mM HCl was added to the protein solution at enzyme-to-substrate (E/S) ratio of 1:50 (w/w) and incubated for 2 h at 37 ºC with mild shaking. Pepsin inactivation was achieved by heating at 85 °C for 5 min and immediately cooled in ice for 5 min. Insoluble solids were removed by centrifugation. The resulting supernatants were adjusted to pH 7.0, to fully inactivate pepsin, and then lyophilized (Ahmed et al., 2015). The CCP and CWP, as well as their hydrolysates (P-CCP and P-CWP), were tested for antimicrobial activity against B.
Table 2: Distribution of Bacillus spp. in examined samples (N=30).
| Type of sample |
Total No. of |
B. cereus | B. subtilis | B. mycoides |
B. licheniformis |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Milk and milk products samples | Household milk | 8 | 26.6 | 3 | 37.5 | 2 | 25 | 1 | 12.5 | 2 | 25 |
| Street vendor milk | 10 | 33.3 | 3 | 30 | 1 | 10 | 3 | 30 | 3 | 30 | |
| Baladi yoghurt | 5 | 16.7 | 1 | 20 | 2 | 40 | 1 | 20 | 1 | 20 | |
| Kareish cheese | 7 | 23.3 | 2 | 28.6 | 2 | 28.6 | 3 | 42.8 | 0 | 0 | |
| Butter | 3 | 10 | 0 | 0 | 3 | 100 | 0 | 0 | 0 | 0 | |
| Rice milk | 11 | 36.6 | 4 | 36.4 | 4 | 36.4 | 1 | 9.1 | 2 | 18.1 | |
| Dairy cattle macroenvironment | Drinking water | 6 | 20.0 | 2 | 33.3 | 0 | 0 | 1 | 16.7 | 3 | 50 |
| Rinse water | 13 | 43.3 | 5 | 38.5 | 4 | 30.7 | 3 | 23.1 | 1 | 7.7 | |
| New bedding material | 11 | 30.6 | 3 | 27.3 | 2 | 18.2 | 4 | 36.3 | 2 | 18.2 | |
| Used bedding material | 24 | 80.0 | 9 | 37.5 | 7 | 29.2 | 5 | 20.8 | 3 | 12.5 | |
| Pasture grass | 16 | 53.0 | 6 | 37.4 | 3 | 18.8 | 3 | 18.8 | 4 | 25 | |
| Pasture soil | 21 | 70.0 | 7 | 33.4 | 5 | 23.8 | 5 | 23.8 | 4 | 19 | |
| Air | 3 | 10.0 | 0 | 0 | 0 | 0 | 2 | 66.7 | 1 | 33.3 | |
| Total (%) | 138 | 100% | 45 | 32.6% | 35 | 25.4% | 32 | 23.2% | 26 |
18.8% |
|
Antimicrobial assay: The liquid-broth method (Ahmed et al., 2020) was used to assess the bactericidal activity of milk proteins hydrolysates against B. cereus at different concentrations. Mid-logarithmic phase cells, grown in brain heart infusion (BHI) broth, were washed and resuspended (to give 6-7 log10 CFU/ml) in 1% trypticase soya broth (TSB), pH 7.3. An aliquot (100 µl) of bacterial suspension was mixed with an equal volume of series of hydrolysates. After incubation at 37 ºC for 24 h, a 30-μl portion was serially diluted in physiological saline and spotted onto a nutrient agar plate. The colony-forming units (CFU) were obtained after incubation at 37 ºC for 18 h.
Result
Bacteriological Analysis of Samples
A total of 180, 30 and 180 milk & milk products, water and environmental samples were screened for the presence of B. cereus, overall 44(24.4%), 6(20%) and 88(48.9%) samples yielded Bacillus spp., respectively. Of these 13(29.5%), 2(33.3%) and 30(34.1%) samples were positive for B. cereus, respectively (Table 2). The highest occurrence% of B. cereus was detected in household milk (37.5%), followed in order by rice milk (36.4%), water (33.3%) and street vendor milk (30%). Moreover, the highest occurrence% of B. cereus in environmental samples was detected in rinse water (38.5%), used bedding material (37.5%), pasture grass (37.5%) and pasture soil (33.3%). Meanwhile, all butter and air samples were devoid of B. cereus (Table 2).
As shown in Table 2, isolates of Bacillus spp. were studied for morphological, biochemical properties and API 50 CHB (Biomerieux, France). These tests were recommended for the identification of Bacillus spp. according to (Varadaraj, 1993; Banykó and Vyletělová, 2009; Bottone, 2010). The identification with classical methods based on morphological and biochemical criteria as well as API techniques showed that Bacillus spp. isolates were identified as Bacillus cereus (B. cereus), Bacillus subtilis (B. subtilis), Bacillus mycoides (B. mycoides) and Bacillus licheniformis (B. licheniformis). Among these isolates, it can be noted that B. cereus was the most frequently occurring Bacillus spp. with an incidence of 32.6% of isolates followed by B. subtilis (25.4%), B. mycoides (23.2%), B. licheniformis (18.8%) (Table 2).
Polymerase Chain Reaction
PCR assay was performed on identified B. cereus isolates using primers designed for virulence genes (Table 3). The hblA, hblD and hblC genes coding for Hemolysin BL (HBL) A, D and C enterotoxigenic gene were demonstrated 42.2, 26.7 and 24.4%, respectively among B. cereus isolates from examined samples. Moreover the nheA, nheB and nheC genes coding for Nonhemolytic (NHE) A, B and C enterotoxigenic gene occurred in 31.1, 33.3 and 24.4%, respectively. A representative gel electrophoresis profile of amplified products of the investigated pathogenic genes was shown in Figure 1.
A total of 45 B. cereus isolates were recovered. Among of them, 33 strains (73.3%) exhibited enterotoxigenic virulent profiles while 12 (26.7%) were non-toxigenic isolates. Six toxin gene profiles were detected in our survey, which covered a total of 45 isolates (Table 4). The most common toxin profile found among the latter isolates was toxin profile 5 (hbl+/nhe+), where strains isolated from most types
Table 6: Log Reduction Values of Disinfectants-Treated Bacillus cereus Spores in suspension at various contact times and temperatures under clean /dirty environment dairy condition.
| A | Exposure duration /temperature | zero |
0.5 hr/20 ºC |
1 hr/20 ºC |
2 hrs/20 ºC |
3 hrs/20 ºC |
4 hrs/20 ºC |
|||||
| Disinfectant | log count cfu/ml |
log reduction cfu/ml |
||||||||||
| clean | dirty | clean | dirty | clean | dirty | clean | dirty | clean | dirty | |||
| Hyperox |
9 log10 |
4 | 2.5 | 5.7 | 3.2 | 6.4 | 4.3 | 6.8 | 4.5 | 7.3 | 4.6 | |
| Cidex | 3.0 | 2.7 | 5.3 | 3.6 | 6 | 4.5 | 6.4 | 4.7 | 6.8 | 4.8 | ||
| Evans Hypochorite | 2.8 | 1.4 | 4 | 2.3 | 4.5 | 2.8 | 4.9 | 3.2 | 5.3 | 3.5 | ||
| Bio Sentry 904 | 1.5 | 1 | 2.7 | 1.3 | 2.9 | 1.7 | 3.4 | 1.9 | 3.7 | 2.3 | ||
| B | Exposure duration /temperature | zero |
0.5 hr/40 ºC |
1 hr/40 ºC |
2 hrs/40 ºC |
3 hrs/40 ºC |
4 hrs/40 ºC |
|||||
| Disinfectant | log count cfu/ml |
log reduction cfu/ml |
||||||||||
| clean | dirty | clean | dirty | clean | dirty | clean | dirty | clean | dirty | |||
| Hyperox |
9 log10 |
4.8 | 2.8 | 6.5 | 3.8 | 6.9 | 4.6 | 7.5 | 4.9 | 7.8 | 5.2 | |
| Cidex | 4.6 | 3.0 | 6.3 | 4.0 | 6.5 | 4.8 | 7.3 | 5.2 | 7.6 | 5.6 | ||
| Evans Hypochorite | 3.5 | 2.0 | 4.8 | 2.7 | 5.3 | 3.2 | 5.6 | 3.6 | 5.9 | 3.9 | ||
| Bio Sentry 904 | 1.9 | 1.3 | 2.9 | 1.8 | 3.3 | 2.3 | 3.8 | 2.7 | 4.3 |
3.4 |
||
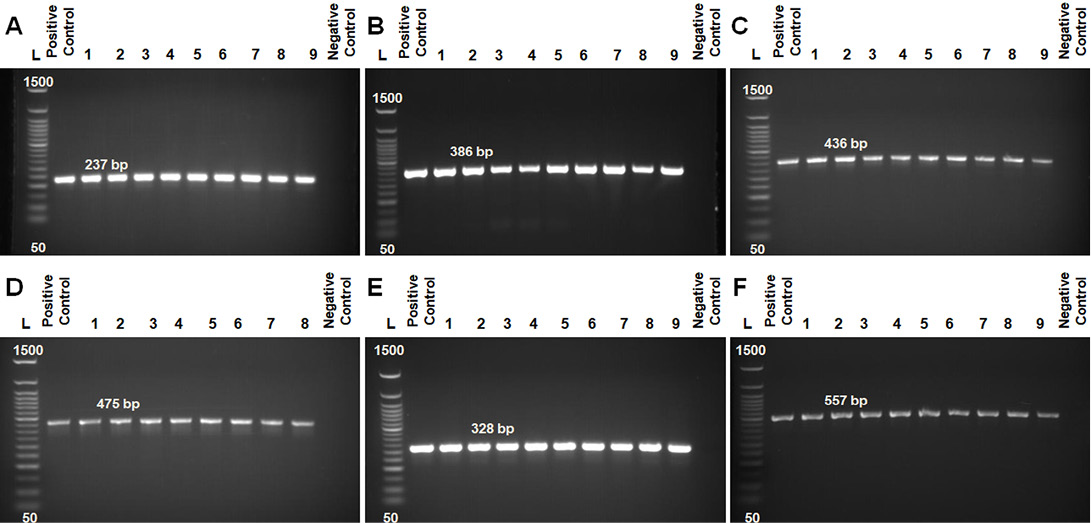
Figure 1: PCR products of amplified of hemolytic non-hemolytic enterotoxin virulent genes identified in B. cereus visualized on agarose gel electrophoresis. The expected molecular size of amplified DNA: 237 bp for hblA gene (A), 386 bp for hblC gene (B), 436 bp for hblD gene (C), 475 bp for nheA gene (D), 328 bp for nheB gene (E) and 557 bp for nheC gene (F). Lane 1-9: samples and Lane (L) DNA ladder 100 bp.
of examined samples were found. Other profiles of less common toxigenic genes were 2 (hbl++/nhe++), 3 (hbl++/nhe+) and 4 (hbl+/nhe++) with four, five and three strains, respectively. The toxigenic gene profile 6 included negative strains for all genes detected (hbl-/nhe-). In almost all profiles, isolated strains of water and milk-related samples were involved.
Antibiogram Pattern of B. cereus Isolates
Antimicrobial susceptibilities for 10 antimicrobial agents commonly used in veterinary clinics and farms were assessed in 45 B. cereus isolates (Table 5 and Figure 2A and B). Irrespective of the origin (milk or milk products, water sources, environmental samples) of the isolates, they were generally resistant to Amoxicillin (68.9%), Ampicillin (62.2%), Penicillin (62.2%), Cephalexin (57.8%) and Tetracycline (51.1%). They were, however, susceptible to other antimicrobials such as Gentamicin (75.6%), Ciprofloxacin (73.3%), Streptomycin (68.9%), Chloramphenicol (66.7%) and Erythromycin (62.2%). The rates of isolates that were resistant to five or more antibiotics from different origins were 61.5% (8/13) of milk-related samples, 50% (1/2) of water sources, 46.7% (14/30) of environmental samples, respectively.
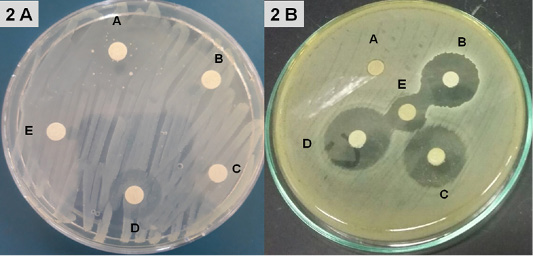
Figure 2: Fig. 2 A from A-E samples of disc diffusion test for Amoxicillin, Ampicillin, Cephalexin, Chloramphenicol, Penicillin; 2 B from A-E samples of disc diffusion test for Tetracycline, Ciprofloxacin, Gentamicin, Streptomycin, Erythromycin.
Antimicrobial Activity
Disinfectants: Upon exposure to Hyperox, B. cereus showed variable log reduction at various contact times under the clean /dirty dairy environment condition. The highest log reduction was 9.32, 10.3 after one /five minutes under clean condition, but an average log reduction of only 5.83, 6.87 after one /five minutes under dirty condition (Figure 3).
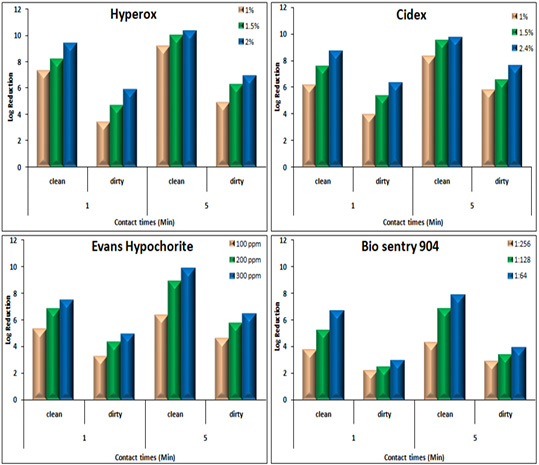
Figure 3: Log Reduction Values of Disinfectants-Treated B. cereus in suspension at various concentrations and contact times under clean /dirty dairy environment condition.
After exposure to CIDEX, B. cereus showed the lowest log reduction at conc.1% under both clean conditions and dirty conditions at the two contact times. The highest log reduction was 9.7 which achieved at conc. 2.4% after 5 minutes exposure under clean condition while log reduction was sharply decreased to 7.56 under the dirty condition at the same concentration.
Hypochlorite showed log reduction of 7.43, 4.89 after 1 min, with an additional average log reduction of 2.41 1.49 following increasing contact times to 5 min under clean and dirty condition respectively at conc. 300 ppm.
In the presence of 4% milk, the efficacy of BioSentry as quaternary ammonium-based disinfectants was mostly affected. Even at the highest con. 1:64, it only reduced the bacterial load by 2.91, 3.88 after 1, 5 minutes respectively. However, it still keeps good activity against B. cereus in the absence of organic matter with log reduction 6.63, 7.84 at 1, 5 minutes using conc. 1:64.
The various disinfectants were more sporicidal in clean than on dirty conditions and in higher temperatures (40 ºC) than lowers (20 ºC) at various contact times. Results showed that Hyperox and Cidex had good sporicidal activity under clean condition with SE values ranging from 7.3-7.8 and 6.8 to 7.6 upon exposure time for 4 hrs at 20 & 40 ºC, respectively while adding milk as organic interfering substance resulted in declining the SE at all the contact times and temperatures with reduction log ranged from 5.2-5.6 and 4.6 to 4.8 (Table 6).
After exposure to Hypochlorite under clean condition, B. cereus spores showed log reduction of 2.8, 4, 4.5, 4.9 and 5.3 /20 ºC, while log reduction increased upon elevating the temperatures to 40 ºC to be 3.5, 4.8, 5.3, 5.6, and 5.9 at exposure times 0.5, 1, 2, 3, 4 hr, respectively. In the presence of organic matter, the Sporicidal efficacy of Hypochlorite was markedly decreased at all contact times at both temperatures. Under the dirty condition, the lowest SE value was 1.4/0.5 hr/ 20 ºC while the highest was 3.9/4hrs/40 ºC.
BioSentry disinfectants exhibited diminished sporicidal activity with SE ranged from 1.0 to 3.4 at 0.5-4hrs hr/20-40 ºC under dirty condition while SE was slightly improved in the absence of organic load to be 1.5 to 4.3 at 0.5-4hrs hr/20-40 ºC.
The kills’ kinetics of Hyperox, CIDEX, Hypochlorite, and BioSentry on B. cereus were shown in Table 6 and Figure 3.
Cow milk proteins hydrolysates: Cow milk proteins and its pepsin hydrolysates were evaluated for their antimicrobial activity against the isolates of recovered B. cereus using a bactericidal assay method as recorded in Figure 4. CCP, CWP and pepsin hydrolysates (P-CCP and P-CWP) were potentially effective in inhibiting B. cereus growth with variable potency and in a dose-dependent manner. Their activity was expressed as log CFU/ml as a function of tested samples concentration incubated with B. cereus for 24 hrs. The intact proteins (CCP and CWP) exhibited little to moderate antimicrobial activity. While P-CWP and P-CCP were very effective against B. cereus resulting in a severe reduction in the CFU of bacteria (ten and eight log10 orders of killing) at a concentration of 2 mg/ml of P-CWP and P-CCP, respectively (Figure 4A and B).
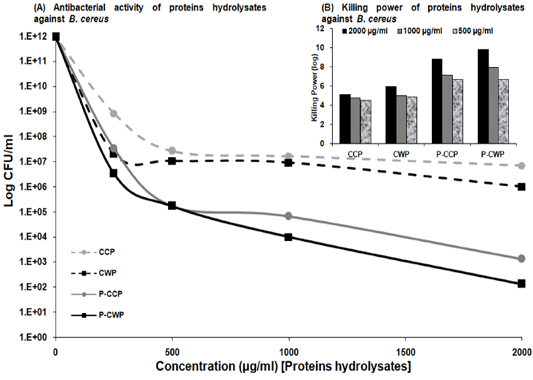
Figure 4: Antibacterial activity of CCP and CWP and its pepsin hydrolysates against B. cereus at different concentrations. The data is presented as log CFU/ml. (Inset) Killing power of algae extracts (2000, 1000 and 500 µg/ml) against Bacillus cereus. The assays were performed in triplicate.
Discussion
The current study revealed the ubiquitous distribution of B. cereus among the investigated dairy environment. Overall of 29.5 and 67% among dairy and environmental samples were positive for B. cereus. Our results are comparable to a similar study that was aimed to isolate B. cereus from a range of farm environments samples include milk, tap water, milking equipment rinse water, grass, and soil. They indicated that the incidence of B. cereus strains in environmental samples of 71.3% was much higher than the found values of 9.8% for raw milk samples (Cui et al., 2016). We agreed with their conclusion that B. cereus was widely distributed within the dairy farm environment with multiple sources for contamination of raw milk.
Our results highlighted that the prevalence of B. cereus in household raw milk was 37.5%. Comparable findings were reported by several studies performed in Ethiopia, Irish, Tunisia, Turkey and Egypt found that the prevalence of B. cereus in raw milk reached 12.86, 23, 47.5, 90.0 and 32%, respectively (Aouadhi et al., 2014; Gundogan and Avci, 2014; Naguib et al., 2014; Garedew et al., 2015; O’Connell et al., 2016).
The occurrence of B. cereus in dairy products including rice milk and street vendor milk were 36.4% and 30%, respectively which agreed well with other studies in Egypt and globally in different studies in USA, Turkey and Brazil reached 17.64, 20 and 24.23%, respectively (Gundogan and Avci, 2014; Montanhini and dos Santos Bersot, 2013; Reis et al., 2013).
B. cereus is a common soil bacterium that is often present in raw milk (Griffiths and Phillips, 1990). The results indicated the occurrences of B. cereus in used bedding material, pasture grass, and pasture soil were 37.5, 37.5, and 33.3%, respectively. Those finding are consistence with the previous study in which the prevalence of B. cereus isolates in bedding, feces, feed, and liquid manure were 93.3, 78.9, 41.2, and 100%, respectively (Cui et al., 2016).
The problem with the presence of B. cereus spores in raw milk is most prominent during the grazing period (Slaghuis et al., 1997) when there is a risk of the teats being contaminated by soil. The spore content of milk was found to be strongly associated with the degree of contamination of the teats with soil (Christiansson et al., 1999). It is very important to prevent the contamination of teats with soil during grazing because B. cereus spore concentrations in soil are approximately 100-fold higher than concentrations in other environmental carriers (feeds, feces, and bedding material) (Vissers et al., 2007).
The earlier study investigated spores of B. cereus in the grass, soil, milking equipment, and raw milk and found a significant positive correlation between the contents of spores in soil, feed, and feces were judged as being important possible sources of raw milk contamination (Labots et al., 1965). Large numbers of Bacillus spores were found in used bedding material (McKinnon and Pettipher, 1983) and bedding was suggested as a participant in the contamination route for B. cereus (Te Giffel et al., 1996; Slaghuis et al., 1997). The soil-milk route is dominant during the grazing of cows, whereas the feed-milk route is dominant during the housing of cows (Slaghuis et al., 1997; Christiansson et al., 1999).
Milking equipment was considered to be an important source of contamination in earlier investigations (Labots et al., 1965; McKinnon et al., 1990). Our findings cleared that the prevalence of B. cereus in milking equipment rinse water was 38.5%. Those results agreed with the former study which mentioned that elevated Bacillus spore contents in the rinse water from the milking equipment were observed and they found a positive correlation of Bacillus spore content between the bedding material and residual milk equipment rinse water (Magnusson et al., 2007).
Overall, the possible sources of raw milk contamination that were considered are soil, feces, bedding, feed, air, and milking equipment (Van Heddeghem and Vlaemynck, 1992). The isolation rates of B. cereus from different sources were high; particularly the detection rate in bedding samples even reached 93.3% (Cui et al., 2016). The most important contamination source found was the bedding material, which contained large numbers of spores and contaminated the milk via contaminated teats. A positive correlation was found between the B. cereus spore content in used bedding material and milk (Magnusson et al., 2007).
According to our results, the air in the barn is not considered a source of raw milk contamination with B. cereus, which agreed well with previous studies which have shown that the number of B. cereus spores in the air was too small to be of major importance source (Te Giffel et al., 1996; Christiansson et al., 1999; Magnusson et al., 2007).
The results clarified that hblA, hblC and hblD were detected in 42.2, 26.7 and 24.4%, respectively and the nheA, nheB and nheC occurred in 31.1, 33.3 and 24.4%, respectively among B. cereus isolates from examined samples. The most common toxin profile found among the latter isolates was toxin profile 5 (hbl+/nhe+), where strains isolated from most types of examined samples were found. The occurrence of hblACD nheABC, observed in this study is consistent with previous studies (Jeßberger et al. 2014; Arslan et al., 2014). Also, the positive rates for nhe (100%) and hbl (55%) in raw milk samples were higher than those found for 54 pasteurized full fat milk samples in China, where the positive rates of nhe and hbl were approximately 62.0 and 37.0%, respectively (Zhou et al. 2008).
The antibiotic resistance profiles of B. cereus isolates are of concern for public health (Cui et al., 2016). All isolates were tested for antimicrobial resistance against 10 antibiotics. B. cereus isolates showed resistance to Amoxicillin, Ampicillin, Penicillin, Cephalexin and Tetracycline with 68.9, 62.2, 62.2, 57.8 and 51.1%, respectively. Multidrug resistance was detected among isolates in 8 milk-related samples, one of the water sources and 14 of environmental samples, respectively. The results of antimicrobials’ susceptibility tests were generally consistent with the previous studies from other countries (Luna et al., 2007; Chaves et al., 2011). B. cereus group strains were found to be sensitive to chloramphenicol, ciprofloxacin, gentamicin, rifampicin, and vancomycin (Luna et al., 2007), and clinical and environmental B. cereus isolates showed a high probability of resistance to erythromycin and tetracycline (Fernandez-Fuentes et al., 2014; Merzougui et al., 2014).
The results indicated that Hyperox and CIDEX have extremely rapid bactericidal activity at higher concentrations 2 and 2.4% after five minutes of exposure times under clean condition resulting in 10.3 and 9.7 log reduction, respectively. Decreasing concentrations and adding organic substances results in the marked decline of the bactericidal activity for both disinfectants. Hypochlorite was still keeping good bactericidal activity against B. cereus at 300 ppm/5minutes under clean condition resulted in 9.82 log reduction, however, after adding 4% milk, the activity decreased which showed 6.38 log reduction.
BioSentry exhibited less potent activity against B. cereus. Even at higher concentrations 1:64/5 minutes, the organic matter sharply hinders its activity which only resulted in 3.88 log reduction. This agreed well with other studies reported that the efficacy of a quaternary ammonium compound (QAC) disinfectant was reduced by two to three logs in the presence of feces as compared to water (Berchieri and Barrow, 1996). Organic matter is one of the most important factors which interfere with the activity of a disinfectant. Disinfectants should be used after thoroughly removing the organic matter as it provides a physical barrier and protects microorganisms from contact with disinfectants (Stringfellow et al., 2009).
B. cereus spores have been isolated from dairy farm sources, including rinse water from the milking equipments, used bedding (Magnusson et al., 2007), teats contaminated with soil, dirty alleys (Christiansson et al., 1999), feces and silage (Vissers et al., 2007). When cows are kept indoors, spores in used bedding are a major source of contamination of milk via contaminated teat and udder surfaces (Magnusson et al., 2007). However, when cows are grazing outdoors, contamination of teats with soil is the main route of milk contamination (Christiansson et al., 1999).
Poor cleaning and disinfection may lead to greater numbers of spores present in the milk (Magnusson et al., 2007). The inactivation of Bacillus spores remains a significant challenge for the food industry (Soni et al., 2016). B. cereus spores are highly resistant to dehydrating and heating treatments and during food preparation, spores may germinate, producing food spoilage or food poisoning (Logan, 2012).
Disinfection of surfaces contaminated with bacterial endospores is not only an issue of bio-security but also an issue faced repeatedly in clinical settings (Rutala et al., 1998). The data highlighted that Hyperox peracetic acid-based disinfectants exhibited high sporicidal activity against B. cereus spores under clean conditions at various contact times at both tested temperatures. The Highest SE value was 7.8 which achieved at 4hrs/40 ºC while the lowest was 4 at 0.5 hr/20 ºC. Even, when 4% of milk was added to the Hyperox solutions, it still showed a satisfactory sporicidal activity at longer contact times with elevated temperatures. At 4hrs/ 20 & 40 ºC under dirty condition, SE values were 4.6 and 5.2, respectively. However, at shorter contact times even at higher temperatures, SE values still low. After 30 minutes of contact time at 20 & 40 ºC, the spores load was reduced only by 2.50 and 2.8 logs, respectively.
CIDEX glutaraldehyde-based disinfectants had a comparable activity to Hyperox on spores of B. Ceruse. CIDEX exhibited lower SE values than Hyperox under clean conditions at various exposure times/temperatures. Under the dirty condition, Cidex showed similar or slightly higher SE values than Hyperox. At 4hrs/ 20 & 40 ºC under dirty condition, SE values were 4.8, 5.6 and 4.6, 5.2 for CIDEX and Hyperox respectively.
In the presence of 4% milk, the efficacy of Sodium Hypochlorite was affected to some extent. It was unable to achieve good SE after 0.5hr /20 ºC under both clean and dirty conditions. Increasing the exposure times to 1, 2, 3 and 4hrs under clean condition/20 ºC, the kill rate got better and the spores load was reduced by 4, 4.5, 4.9 and 5.3 logs respectively. Under dirty condition the efficacy was markedly reduced after 1, 2, 3 and 4hrs, The SE was 2.3, 2.8, 3.2, and 3.5 log kill was observed. Elevating temperatures to 40 ºC resulted in improving the efficacy at all the various contact times under both clean and dirty conditions. However, organic load still represents an obstacle even after rising the temperatures /40 ºC, the efficacy was still low after 1, 2, 3 and 4hrs, with SE was 2.7, 3.2, 3.6, and 3.9 log reductions which highlighted the importance of cleaning and sanitation procedures to eliminate the organic load before the application of the chemical disinfectants.
Quaternary ammonium-based BioSentry disinfectants showed the lowest sporicidal efficacy against B. cereus spores at all contact times under both clean and dirty conditions. Even after increasing the exposure times and elevating the temperatures, an only slight improvement in the efficacy with minor addition log reduction was observed. We assumed that B. cereus spores had a more resistant ability to BioSentry than other disinfectants.
Protecting human health from the harmful effects of pathogenic B. cereus has become extremely challenging. The adverse effects of chemical preservatives on human health, their limited application, susceptibility, toxicity, and microbial resistance increase the demand to search for potentially effective, healthy, safer and natural antimicrobial agents with a unique mechanism of action against the dreadful pathogens. Thus, antimicrobial activity of cow milk proteins and their pepsin hydrolysates can provide a key aspect of treatment of B. cereus infections than traditional antimicrobial agents cannot provide, and be used as natural preservatives to ensure healthy and safe food without the unpleasant side effects of chemical preservatives. We have shown that pepsin hydrolysates of cow milk proteins exerted strong bactericidal activity, dose-dependently, against B. cereus. Notably, B. cereus killing by P-CWP and P-CCP were observed at concentrations as low as 2 mg/ml. P-CWP and P-CCP were potentially effective against tested pathogens with variable potency (ten and eight log10 orders of killing).
Various studies evaluated the antimicrobial activities of the cow milk proteins hydrolysates viz., P-CWP and P-CCP. Different enzymes viz., pepsin, trypsin, α-chymotrypsin, enzymes of animal, microbial, or plant origin (papain, alcalase, flavourzyme, pronase, ficin, thermolysin and neutrase) are used to break large polypeptides into specific small peptides to investigate its antimicrobial activity against both Gram-positive and Gram-negative bacteria including B. cereus and B. subtilis (Benkerroum, 2010; Abd El-Fattah et al., 2017; El-Sayed and Awad, 2019). Antimicrobial activity of pepsin hydrolysates of milk proteins has been reported with clear inhibitory effects on various pathogenic cultures especially B. cereus (Almaas et al., 2011; Mohanty et al., 2014; Kumari and Vij, 2015).
Conclusion
The milking environment in the household rearing of cattle in Sohag governorate, Egypt was deemed to be unsafe. High humidity, mud and animal excretion were present in all milking areas. Before, during and after milking, the skin of the udder and milkers’ hands were not cleaned or disinfected. There is lacking of the basic requirements and sanitary measures. The milk was transported and distributed from farmers’ houses to local dairy shops or consumers without cooling or heat treatment. The practices described could lead to contamination of raw milk and milk products on the farm with B. cereus. Other sources of contamination are contaminated water, udder infections and environmental contamination in cow’s house environment and local dairy shops. Strict sanitary measures and disinfection practices should be followed to reduce the initial raw water-milk contamination level to avoid subsequent recontamination of dairy products. Close monitoring and adhering to the national and international regulation of antibiotic usage is crucial to minimize the amplification and dissemination of resistant strains.
Acknowledgement
The authors would like to acknowledge South Valley University, Qena, Egypt for supports.
Conflict of Interest
No conflicts of interests are announced by authors.
Authors Contribution
All authors contributed in writing of this research article. All authors contributed in collection of samples and traditional identification of B. cereus, and contributed in molecular characterization of isolated strains, antimicrobial activity of peptides and disinfectant.
References