Advances in Animal and Veterinary Sciences
Research Article
Bacteriological, Hematological and Biochemical Diagnostic Studies on Diarrheic Arabian Horse Foals Caused by Enterobacterial Infections
Emad Beshir Ata1, Soad M. Nasr1*, Amany M. Mohamed1, Tamer H. Abd El-Aziz1, Ehab A. Fouad2, Doaa Sedky1, Somia A. Nassar1,3, Alaa A. Ghazy1
1Department of Parasitology and Animal Diseases, National Research Centre, 33 Bohouth St., Dokki, 12622 Giza, Egypt; 2Department of Microbiology and Immunology, National Research Centre, 33 Bohouth St., Dokki, 12622 Giza, Egypt; 3Department of Medical Laboratory Science, College of Applied Medical Science, Prince Sattam bin Abdul Aziz University, Wadi Addawasir, Kingdom of Saudi Arabia.
Abstract | This study was conducted to evaluate the most important enteric bacteria causing diarrhea in Arabian horse foals and its impact on blood parameters. Identification and sensitivity tests for bacterial isolates from diarrheic foals were carried out. Complete blood counts and serum biochemical constituents were also investigated. Blood (with and without EDTA-K3), rectal swabs and fecal samples were collected from both 27 diarrheic and 73 apparently healthy horse foals located in an Arabian horse farm in Cairo, Egypt, during a period extending from 2018 to 2019. Bacteriological examination for rectal swabs was carried out using different selective and specific media followed by different routine biochemical tests. It revealed the isolation of 140 bacterial isolates, of which 120 were Gram negative enterobacterial isolates and the rest were Gram positive bacterial isolates. Enterobacter aerogens (27 isolates) was found to represent the major causative agent of diarrhea in foals among the enterobacterial isolates, followed by Proteus mirabilis (18 isolates), E. coli (10 isolates), Citrobacter diversus (9 isolates), Salmonella enterica (4 isolates) and Proteus vulgaris (2 isolates). Ciprofloxacin and gentamicin were the most effective against enterobacterial species isolated from diarrheic foals followed by amikacin, neomycin and rifamycin. In diarrheic foals, the values of RBCs, total WBCs, lymphocytes and platelets counts, and hematocrit values were markedly (P<0.05) increased, and microcytic normochromic anaemia was recorded. In diarrheic foals, serum total proteins, albumin, and total globulins; β2-globulin, γ-globulin, urea, calcium and sodium levels were markedly (P<0.05) decreased, while the α2-globulin, AST activity and creatinine level were significantly (P<0.05) increased. In conclusion, the major causative agents of diarrhea in the examined foals were Enterobacter aerogens. The most effective antibacterial agents were ciprofloxacin and gentamicin against Gram negative enterobacterial species isolated from diarrheic foals. In addition, hematological, metabolic and electrolytes’ disturbances were recorded in diarrheic foals.
Keywords | Diarrhea, Arabian foals, Enterobacterial infections, Hemogram, Biochemical parameters
Received | January 04, 2020; Accepted | March 12, 2020; Published | March 25, 2020
*Correspondence | Soad M. Nasr, Department of Parasitology and Animal Diseases, Veterinary Research Division, National Research Centre, 33 Bohouth Street, Dokki, P.O. Box 12622, Giza, Egypt; Email: soadnasr@yahoo.com
Citation | Ata EB, Nasr SM, Mohamed AM, El-Aziz THA, Fouad EA, Sedky D, Nassar SA, Ghazy AA (2020). Bacteriological, hematological and biochemical diagnostic studies on diarrheic arabian horse foals caused by enterobacterial infections. Adv. Anim. Vet. Sci. 8(4): 412-421.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.412.421
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ata et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Foal diarrhea is a worldwide major problem in equines and is reported to be the most common cause of death in young age (Barr, 2018; Haq et al., 2018). The affected animals show diarrhea, inappetance, and poor body condition as major clinical signs. While in some per-acute cases, high fever, leukopenia, increase heart rate, anorexia and septic shock were detected (Haq et al., 2017a). It is recorded that nearly 80% of the foals suffered from diarrhea at least one time during their life (Frederick et al., 2009). A single causative agent is often not found (Schoster et al., 2015). However, coinfection between infectious agents was recorded as being more prevalent and may result in more severe gastrointestinal disorders (Slovis et al., 2014). In newly born foals, the infectious causes of diarrhea are mainly of bacterial origin, while viral or parasitic organisms are less common (Frederick et al., 2009). In Pakistan, it has been revealed that 65.7% of the examined diarrheic foals were due to bacterial infection (Haq et al., 2018).
Various reports recorded that the most common bacterial pathogens isolated from infected diarrheic cases were Salmonella spp. (Juffo et al., 2017), E. coli, and Clostridium spp. (Haq et al., 2018). The Gram negative bacteria are among the most important bacterial pathogens incriminated in the etiology of the gastrointestinal infections in horses (El-Baroudy, 1987). The family Enterobacteriaceae may cause great dangerous gastrointestinal disorders. The most commonly recognized causes of neonatal foal diarrhea are Escherichia coli, Salmonella Typhimurium, Shigella spp. and Citrobacter spp. Hemolytic E. coli strain was isolated from the feces of neonatal diarrheic foals (Holland et al., 1996).
The treatment strategies of diarrheic cases should provide supportive care (mainly fluid therapy) and a specific antimicrobial treatment (Mallicote et al., 2012). Any diarrheic foal less than 30 days old should take a broad-spectrum antibiotic because of the high incidence of bacteraemia (Frederick et al., 2009). However, the uncontrolled use of antimicrobials has resulted in a devastating antibiotics resistance problem which spread all over the world (Singh, 2009). A comprehensive study carried out by Haq et al. (2017a) in Pakistan revealed that the obtained isolates (92%) were resistant to three or more antibiotics. They also found that the highest resistance (86%) was against sulphamethoxazole (23.75 mg), while the lowest (4%) was against trimethoprim (5 mg).
Laboratory assays for hematological, and biochemical parameters are good guide for diagnosis and treatment of horse diseases. In diarrheic animals, dehydration is resulted from loss of body fluid which is accompanied by decreases in the extracellular fluid volume with increases in intracellular fluid volume. So, the hematocit is the important marker to detect the change in the extracellular fluid volume (Weiss and Wardrop, 2010). The mortality from diarrhea is usually results from dehydration, acidosis and loss of electrolytes (Radostits et al., 2007). In diarrheic foals, hyponatremia is the most common electrolyte derangements and is frequently related to diarrhea (Wong et al., 2007).
Therefore, this study was performed to evaluate the most important enteric bacteria causing diarrhea in Arabian horse foals by identification of bacterial isolates in diarrheic foals compared with the apparently healthy ones. The sensitivity of antibacterial agents against the obtained bacterial isolates was also assessed. In addition, the impact of diarrhea caused by enterobacterial infections on complete blood counts and serum biochemical parameters of diarrheic Arabian horse foals were also investigated.
MATERIAL AND METHODS
Ethics statement
This study was carried out according to the guidelines of the Institutional Animal Care and Use Committee at National Research Centre, Giza, Egypt, and the Protocol Approval No. 18/102.
Animals used
A total number of 100 Arabian horse foals (27 diarrheic and 73 apparently healthy), aged 1 to 8 weeks old, located in an Arabian horse farm in Cairo, Egypt during a period extended from 2018 to 2019 were used in this study. The diarrheic foals exhibited profuse persistent diarrhea accompanied with increase of body temperature, inappetance, depletion, depression, and colic, while some cases died suddenly. Multiple data about age, severity of the case, nature of the diarrhea, body temperature, type and response to the used antibiotics, and the administrated anthelmintics protective programme were collected using a questionnaire.
Sampling
Rectal swab samples
One hundred rectal swab samples were collected from animals using Brain Heart Infusion (BHI) broth -as the transport medium and stored in icebox at 4–8°C and then transported to the laboratory for bacteriological examination.
Fecal samples
Fresh fecal samples were collected for parasitological examination using a combined sedimentation-flotation technique (Becker et al., 2016) and fecal smears were also stained by a modified Ziehl–Neelsen technique for detection of oocysts of Cryptosporidium spp. (Soufy et al. 2017).
Blood samples
Two blood samples were collected from jugular vein of each of the clinically healthy and the diarrheic foals, one into vacutainer tube containing ethylenediaminetetraacetic acid tripotassium (EDTA-K3) as anticoagulant for hematological investigation and the other sample was collected into plain vacutainer tube without anticoagulant agent for sera separation. The serum were kept at -20 °C for further biochemical analyses.
Bacteriological examination
The collected rectal swabs were firstly inoculated into brain heart infusion broth (Oxoid, UK), incubated at 37°C for 16-18 h for the primary enrichment of bacterial isolates. The obtained growth was subcultured onto three media; sheep blood agar with 5% (v/v) defibrinated sheep blood, MacConkey’s agar (Oxoid, UK) and mannitol salt agar followed by incubation at 37°C for 24 h. Representative colonies from culture positive plates were subcultured on nutrient agar for isolation of pure colonies. Colony morphology, colour, size, elevation, the status of hemolysis were recorded. Pure colonies were transferred to nutrient agar slants for further biochemical tests as oxidase, nitrate, urease, IMVic, catalase, TSI (triple sugar iron agar), coagulase, indol, methyl red, Voges-Proskaur, citrate and sugar fermentation These culture isolates were subjected to Gram’s staining procedure and examined microscopically under oil emersion lens (1000× magnification). Diagnostic oxidase discs (bioMerieux, France) were used for determination of the presence of oxidase enzyme to differentiate between the oxidase negative Enterobacteriaceae and other Gram negative oxidase positive bacteria (Quinn et al., 2013). Biochemical tests were used to differentiate between Proteus vulgaris and Proteus mirabilis. The differentiation was performed by Indol test which was negative for P. mirabilis and positive for Proteus vulgaris (Quinn et al., 2013; Al-Jumaily and Zgaer, 2016). An anaerobic bacteriological examination was carried out according to Skariyachan et al. (2010).
Antibacterial sensitivity test
A total of fourteen antibacterial discs (Oxoid, UK) were used in this study. They included Amikacin 30 μg (AK30), Ampicillin10 μg (AM10), Augmentin (Amoxicillin/ Clavulanic acid) 30 μg (AMC30), Ciprofloxacin 5 μg (CIP5), Clindamycin 2 μg (DA2), Erythromycin 15 μg (E15), Gentamicin 10 μg (CN10), Neomycin 30 μg (N30), Novobiocin 30 μg (NV30), Oxacillin 1 μg (OX1), Oxytetracycline 30 μg (OT30), Rifamycin 30 μg (SV30), Tetracycline 30 μg (TE30) and Trimethoprim/sulfamethoxazole 25 μg (SXT25). Disc diffusion method using Muller Hinton agar was performed to determine the antibiotic sensitivity of all bacterial isolates according to the Clinical and Laboratory Standard Institute (CLSI) recommendations (CLSI, 2016, 2019). The degree of sensitivity was determined by measuring the zone of growth inhibition produced by the diffusion of the antibiotic into the surrounding medium after the incubation at 37 °C for 24 h under aerobic conditions. The results were interpreted according to the methods of Kassim et al. (2016) and Sahu et al. (2018).
Hematological examination
Complete blood pictures for healthy and diarrheic foals were evaluated using a hematological analyzer (Exigo Vet, Sweden). The hematological examinations included erythrogram [Erythrocytes (RBCs), hematocrit (HCT), hemoglobin (Hb) concentration, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC)], and leukogram [total and differential leukocytic counts (lymphocytes, monocytes, neutrophils, eosinophils and basophils)], and platelets counts.
Serum biochemical analyses
Total serum proteins levels were determined spectrophotometrically using commercial test kits from bioMérieux, France. Protein electrophoresis was carried out using SAS-1 SP-24 Kit -a semi-automated agarose gel electrophoresis (AGE) system- (Helena Laboratories, Helena Biosciences, Gateshead, UK) according to the method of the manufacturer. Using the computer software Phoresis (Helena Biosciences), electrophoretic curves plus related quantitative specific protein concentrations for each sample were displayed. Relative protein levels within each fraction were determined as the optical absorbance percentage, and absolute concentrations (g/dl) were calculated using the total serum proteins concentration.
Alanine transaminase (ALT) and aspartate transaminase (AST) activities, the concentrations of urea, and creatinine were measured in the foals’ serum spectrophotometrically using commercial test kits purchased from Erba Mannheim, Germany.
Serum calcium and inorganic phosphorous levels were determined spectrophotometrically using commercial test kits purchased from Centronic GmbH, Germany.
The biochemical parameters were determined using Double Beam UV/Visible spectrophotometer, Model T80, UK.
Serum sodium and potassium were determined using Roche Modular analyzers (Roche Diagnostics, COBAS C702, Germany). However, Chloride was determined using ADVIA Chemistry XPT System (Siemens Healthineers, Germany).
Statistical analysis
All data were subjected to statistical analysis and presented as mean±standard error (Mean ± SE). The difference between diarrheic and non-diarrheic groups of foals was evaluated for significance using Student t-test (Petrie and Watson, 2013) using Statistical Package for Social Science (SPSS) software version 17 computer program (SPSS Inc, Chicago, IL, USA). Differences were considered significant at P<0.05.
RESULTS
Bacteriological findings
Bacteriological findings for rectal swabs
Bacteriological examination of 100 rectal swab samples obtained from 27 diarrheic foals and 73 apparently healthy ones revealed the isolation of 140 bacterial isolates, from which 120 were Gram negative enterobacterial isolates and 20 were Gram positive bacterial isolates (Table 1).
The Gram negative enterobacterial isolates exhibited the typical Gram negative medium-sized bacilli, showing negative oxidase by oxidase test. These oxidase negative bacilli were further differentiated into lactose fermenting (pink colonies) and non-lactose fermenting (colorless colonies) genera using MacConky’s agar and biochemical tests as Indole, methyl red, Voges-Proskauer, citrate, TSI, catalase, nitrate. The detected lactose fermenting genera were Escherichia, Enterobacter and Citrobacter (which could be differentiated by IMVic and urease test), while the non-lactose fermenting genera were Salmonella and Proteus (which could be differentiated by urease test). Genus Proteus was firstly identified with their characteristic swarming phenomena on blood agar. Moreover, pale color on MacConky’s agar because inability of Genus Proteus to ferment lactose sugar. In addition to, biochemical tests were used to differentiate between Proteus vulgaris and Proteus mirabilis. Both were catalase positive, urease positive, oxidase negative, Vogas-Proskauer negative, urease positive and methyl red positive. Indol test was negative for P. mirabilis and positive for Proteus vulgaris. Regarding to the biochemical reactions and colonial differentiation, the Gram negative enterobacterial isolates (120 isolates) in both diarrheic and apparently healthy foals were categorized into six species; which were Escherichia coli (45 isolates), Proteus mirabilis (20 isolates), Enterobacter aerogens (30 isolates, 38.57%), Citrobacter diversus (9 isolates), Proteus vulgaris (12 isolates) and Salmonella enterica (4 isolates).
Gram positive bacterial isolates (20 isolates) exhibited the typical Gram positive cocci. On nutrient agar; S. aureus colonies appeared golden yellow (yellow and cream to buff varieties), smooth, opaque, circular and medium in size, while Streptococcus spp. colonies are small in size, 1-2 mm diameter (dew drop). Moreover, on blood agar; S. aureus colonies were surrounded by zone of ß-hemolysis, while S. epidermidis is non hemolytic. S. aureus on mannitol salt agar; colonies were yellow in colour surrounded by yellow halo with yellow colored medium. The non-hemolytic colonies of S. epidermidis on blood agar, showed pink colonies on mannitol salt agar. Gram positive cocci arranged in clusters like bunch of grapes in staphylococci, Micrococcus spp. arranged in tetrad, while Streptococcus spp. arranged in long or short chain like sting of bead. The Gram positive cocci were differentiated by culture character, catalase test, coagulase test and other biochemicals (as citrate, nitrate, oxidase, Indole, methyl red, Voges-Proskauer, urease, sugar fermentation as glucose, sucrose, mannitol, maltose) into the following four species; S. aureus (7 isolates), S. epidermidis (4 isolates), Micrococcus spp. (6 isolates) and Streptococcus spp. (3 isolates) which were detected only in non-diarrheic apparently healthy foals but not in diarrheic foals (Table 1).
Prevalence of enterobacterial infections in the diarrheic foals
Enterobacter aerogens (27 isolates, 38.57%) was found to represent the major causative agent of diarrhea in Arabian foals among the Gram negative enterobacterial isolates, followed by Proteus mirabilis (18 isolates, 25.72%), E. coli (10 isolates, 14.28%), Citrobacter diversus (9 isolates, 12.86%), Salmonella enterica (4 isolates, 5.72%) and Proteus vulgaris (2 isolates, 2.85%). There were no any Gram positive bacteria isolated from the studied diarrheic foals (Table 1). Moreover, anaerobic bacteriological examination revealed no bacterial isolates of anaerobes were detected.
Prevalence of enteric bacteria in diarrheic foals compared with non diarrheic apparently healthy foals
Results of the prevalence of Gram negative enteric bacteria in the diarrheic foals compared with the non diarrheic apparently healthy foals revealed that Enterobacter aerogens (27 isolates, 38.57% versus 3 isolates, 4.85%), Proteus mirabilis (18 isolates, 25.72% versus 2 isolates, 2.85%), Citrobacter diversus (9 isolates, 12.86% versus no isolates), Salmonella enteric (4 isolates; 5.72% versus no isolates), E. coli (10 isolates; 14.28% versus 35 isolates; 50%), and Proteus vulgaris (2 isolates, 2.85% versus 10 isolates, 14.28%) were the most frequent enteric pathogens, respectively. Meanwhile, Gram positive bacteria including S. aureus, S. epidermidis, Micrococcus spp. and Streptococcus spp. were detected only in non diarrheic apparently healthy foals (Table 1).
Antibacterial sensitivity test for the identified strains
The most effective antibacterial agents against Gram negative enterobacterial species were ciprofloxacin and gentamicin followed by amikacin, neomycin and rifamycin. Moreover, most of the isolated enteric bacteria showed resistance against ampicillin and erythromycin (Table 2).
Parasitological findings
The parasitological examination revealed absence of parasitic helminths and protozoa infections (Cryptosporidium oocysts) in the fecal samples.
Hematological findings
Complete blood counts were performed in diarrheic and clinically apparently healthy foals and are illustrated in Table 3.
In diarrheic foals, the result revealed marked (P<0.01) increase in RBCs count and HCT level, while MCV and
Table 1: Bacterial isolates from both diarrheic and apparently healthy Arabian horse foals.
| Bacterial isolates | Apparently healthy foals (70 isolates) | Diarrheic foals (70 isolates) | Total (140 isolates) | |||
| No. | % | No. | % | No. | % | |
| Gram negative enterobacteria | ||||||
|
Escherichia coli |
35 | 50 | 10 | 14.28 | 45 | 32.14 |
| Proteus mirabilis | 2 | 2.85 | 18 | 25.72 | 20 | 14.28 |
| Enterobacter aerogens | 3 | 4.28 | 27 | 38.57 | 30 | 21.44 |
| Citrobacter diversus | - | - | 9 | 12.86 | 9 | 6.44 |
| Proteus vulgaris | 10 | 14.28 | 2 | 2.85 | 12 | 8.57 |
| Salmonella enterica | - | - | 4 | 5.72 | 4 | 2.85 |
| Total isolates | 50 | 70 | 120 | |||
| Gram positive enterobacteria | ||||||
| Staphylococcus aureus | 7 | 10 | - | - | 7 | 5.00 |
| Staphylococcus epidermidis | 4 | 5.72 | - | - | 4 | 2.85 |
|
Micrococcus spp. |
6 | 8.58 | - | - | 6 | 4.28 |
|
Streptococcus spp. |
3 | 4.29 | - | - | 3 | 2.15 |
| Total isolates | 20 | - | 20 | |||
Table 2: Susceptibility of the isolated bacteria (140 isolates) to the selected fourteen antibacterial agents using the antibacterial sensitivity test.
| Antibacterial Agents | Code | Conc. |
E. coli |
Proteus mirabilis | Enterobacter aerogenes | Citrobacter diversus | Proteus vulgaris | Salmonella enterica | Staph. aureus |
|||||||||||||
| S | R | S | R | S | R | S | R | S | R | S | R | S | R | |||||||||
| Amikacin | AK | 30 | 22 | 23 | 20 | - | 30 | - | 7 | 2 | 12 | - | 4 | - | 7 | - | ||||||
| Ampicillin | AM | 10 | 16 | 29 | 2 | 18 | 10 | 20 | 9 | - | 12 | - | 4 | - | 6 | 1 | ||||||
| Augmentin | AMC | 30 | 28 | 17 | 5 | 15 | 7 | 23 | 7 | 2 | 12 | - | 4 | - | 5 | 2 | ||||||
| Ciprofloxacin | CIP | 5 | 45 | - | 20 | - | 30 | - | 9 | - | 12 | - | 4 | - | 7 | - | ||||||
| Clindamycin | DA | 2 | 35 | 10 | 4 | 16 | 8 | 22 | 9 | - | 10 | 2 | 4 | - | 7 | - | ||||||
| Erythromycin | E | 15 | 5 | 40 | 5 | 15 | 5 | 25 | 5 | 4 | 10 | 2 | 4 | - | 5 | 2 | ||||||
| Gentamicin | CN | 10 | 45 | - | 20 | - | 30 | - | 9 | - | 12 | - | 4 | - | 7 | - | ||||||
| Neomycin | N | 30 | 18 | 27 | 20 | - | 30 | - | 8 | 1 | 12 | - | 4 | - | 7 | - | ||||||
| Novobiocin | NV | 30 | 17 | 28 | 20 | - | 7 | 23 | 7 | 2 | 12 | - | 4 | - | 7 | - | ||||||
| Oxacillin | OX | 1 | 25 | 20 | 3 | 17 | 7 | 23 | 8 | 1 | 10 | 2 | 4 | - | 7 | - | ||||||
| Oxytetracycline | OT | 30 | 37 | 8 | 4 | 16 | 12 | 18 | 9 | - | 12 | - | 4 | - | 4 | 3 | ||||||
| Rifamycin | SV | 30 | 39 | 6 | 20 | - | 15 | 15 | 9 | - | 12 | - | 4 | - | 4 | 3 | ||||||
| Tetracycline | TE | 30 | 35 | 10 | 7 | 13 | 18 | 12 | 9 | - | 12 | - | 4 | - | 3 | 4 | ||||||
| TMP/SMZ | SXT | 25 | 42 | 3 | 2 | 18 | 14 | 16 | 7 | 2 | 10 | 2 | 4 | - | 6 | 1 | ||||||
S: Sensitive; R: Resistance; Augmentin: (Amoxicillin/ Clavulanic acid). TMP/SMZ: rimethoprim/sulfamethoxazole; Conc.: Concentration (µg/Disc). E. coli: Escherichia coli; Staph. aureus: Staphylococcus aureus.
Table 3: Hematological pictures of diarrheic and apparently healthy Arabian horse foals. (Mean ± SE, N=5).
| Parameters | Apparently healthy Arabian foals | Diarrheic Arabian foals |
|
Red blood cell counts (×106/µl) |
8.83 ± 0.13 | 10.46 ± 0.11** |
| Hematocrit (%) | 34.20 ± 1.07 | 36.94 ± 1.05** |
| Hemoglobin (g/dl) | 11.70 ± 0.28 | 12.05 ± 0.46 |
| Mean corpuscular volume (fl) | 38.63 ± 0.89 | 34.38 ± 0.64** |
| Mean corpuscular hemoglobin (pg) | 13.20 ± 0.18 | 11.72 ± 0.37** |
| MCHC (g/dl) | 34.33 ± 0.35 | 33.97 ± 0.44 |
|
Platelets (×103/µl) |
173.33 ± 11.44 | 226.92 ± 11.08** |
|
Total leukocytes (×103/µl) |
5.45 ± 0.37 | 6.78 ± 0.32* |
|
Lymphocytes (×103/µl) |
1.17 ± 0.07 | 1.47 ± 0.04* |
|
Monocytes (×103/µl) |
0.43 ± 0.07 | 0.47 ± 0.02 |
|
Neutrophils (×103/µl) |
3.93 ± 0.57 | 4.14 ± 0.34 |
|
Eosinophils (×103/µl) |
0.57 ± 0.07 | 0.69 ± 0.03 |
* Significant difference at P< 0.05. ** Significant difference at P< 0.01. MCHC: Mean corpuscular hemoglobin concentration.
Table 4: Serum biochemical constituents of diarrheic and apparently healthy Arabian horse foals. (Mean ± SE, N=5).
| Parameters | Apparently healthy Arabian foals | Diarrheic Arabian foals |
| Total proteins (g/dl) | 5.14 ± 0.03 | 4.41 ± 0.15* |
| Albumin (g/dl) | 3.33 ± 0.01 | 2.40 ± 0.06* |
| Total globulins (g/dl) | 2.47 ± 0.00 | 2.05 ± 0.12* |
|
α1-globulin (g/dl) |
0.57 ± 0.02 | 0.44 ± 0.09 |
|
α2-globulin (g/dl) |
0.24 ± 0.02 | 0.50 ± 0.10* |
|
α1-globulin (g/dl) |
0.47 ± 0.01 | 0.46 ± 0.05 |
|
α2-globulin (g/dl) |
0.30 ± 0.00 | 0.23 ± 0.01* |
|
α-globulin(g/dl) |
0.83 ± 0.03 | 0.55 ± 0.09** |
| Alanine amino transferase (IU/l) | 9.31 ± 0.18 | 8.73 ± 0.26 |
| Aspartate amino transferase (IU/l) | 96.32 ± 1.89 | 178.28 ± 8.80** |
| Urea (mg/dl) | 41.45 ± 0.36 | 18.14 ± 1.06** |
| Creatinine (mg/dl) | 0.52 ± 0.01 | 0.64 ± 0.03* |
| Calcium (mg/dl) | 12.43 ± 0.45 | 11.24 ± 0.21* |
| Inorganic phosphorus (mg/dl) | 4.81 ± 0.20 | 4.01 ± 0.30 |
| Sodium (mEq/l) | 143.80 ± 0.20 | 136.2 ± 2.80** |
| Potassium (mEq/l) | 4.30 ± 0.03 | 4.25 ± 0.11 |
| Chloride (mEq/l) | 101.00 ± 1.22 | 101.25 ± 0.37 |
* Significant difference at P< 0.05. ** Significant difference at P< 0.01.
MCH levels significantly (P<0.01) decreased compared with the apparently healthy foals. No statistical significance was recorded in Hb concentration and MCHC values between both groups. The total leukocytes count significantly (P<0.05) increased in diarrheic foals accompanied with marked (P<0.05) increase in lymphocytes in comparison with healthy ones. There was non-significant increase in the counts of neutrohpils, eosinophils, and monocytes in diarrheic foals than healthy ones. Platelets count significantly (P<0.01) increased in diarrheic foals in comparison with healthy foals. (Table 3).
Serum biochemical findings
The results of biochemical parameters are shown in Table 4 and Figure 1.
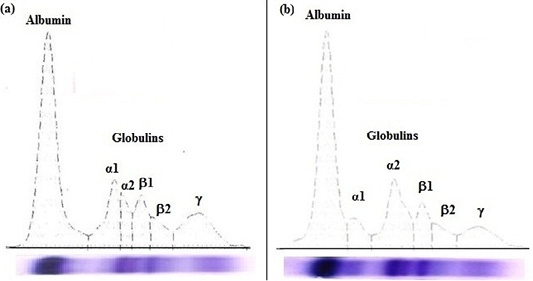
Figure 1: Agarose gel electrophoregram of serum proteins: (a) apparently healthy horse foal and (b) diarrheic Arabian horse foal.
In diarrheic horse foals, serum total proteins, albumin, and total globulins; β2-globulin and γ-globulin levels were significantly (P<0.05) lower, while the α2-globulin was markedly (P<0.05) higher than clinically apparently healthy foals. No statistical significant difference in the levels of α1-globulin and β1-globulin were recorded in the serum of both groups (diarrheic and healthy foals).
In diarrheic foals, serum AST activity and creatinine level markedly (P<0.05) increased, however, serum urea level markedly (P<0.01) decreased compared with healthy foals.
The result of macro-elements and electrolytes revealed that diarrheic foals showed significant (P<0.05) decreases in serum calcium and sodium levels, however no significant alterations was noticed in serum inorganic phosphorus, potassium, and chloride levels compared with healthy ones.
DISCUSSION
Foals mortality due to diarrhea causes a great economic loss in stud-horse farms and the world horse industry. It causes severe impact on farmer’s livelihood (Haq et al., 2017b), especially during the first six months of life through which up to 60% of foals suffered from diarrhea (Schoster et al., 2017). Outbreaks of diarrhea in foals represent the worst syndrome among disease problems in Arabian horse farms in Egypt. Variable causes of this syndrome render the medical approach very difficult.
The animals under study were suffering from clinical signs mainly diarrhea, anorexia, and fever, which were previously recorded by Juffo et al. (2017). The diarrhea is a result of bacterial endotoxins which are mediated by hyperstimulation and the inflammatory response of the host (Oliver and Stämpfli, 2006). Isolation and identification of the bacterial causative agent is of high importance as some bacteria are zoonotic. So, it could be a biological hazard for the human being. It is recorded that infectious causes of diarrhea in newly born foals are mainly of bacterial origin, while viral or parasitic organisms are less common (Frederick et al., 2009).
The current study revealed the predominance of Gram negative bacteria in both healthy and diarrheic foals. Bacteria were recovered at a higher rate from diarrheic foals than from apparently healthy animals. The obtained results clarified that Enterobacter aerogens (27 isolates, 38.57%) were found to represent the major causative agent of diarrhea followed by Proteus mirabilis (18 isolates, 25.72%), E. coli (10 isolates, 14.28%), Citrobacter diversus (9 isolates, 12.86%), Salmonella enterica (4 isolates, 5.72%) and Proteus vulgaris (2 isolates, 2.85%). Meanwhile, the results of identification of bacteria from diarrheic foals in Pakistan revealed the presence of variable causative agents; mainly E. coli was the most (48.77%), followed by Clostridium perfringens (18.56%) and Salmonella spp. (17.9%) (Haq et al., 2017b; Haq et al., 2018). Proteus mirabilis can be assumed as a cause of diarrhea as it has been detected in many equine respiratory cases (Lakritz et al., 1993) and foal morality (Veronesi et al., 2006). Citrobacter spp. was isolated from several cases, either died from colic and severe enteritis or diseased horses showing diarrhea (El-Baroudy, 1987).
The present results indicated that there is a great variation between the diarrheic and healthy foals in the type and number of Gram negative isolates. Previous results clarified that Escherichia coli fimH (30% versus 25%), Salmonella spp. (25% versus 7%), Strongyloides westeri (25% versus 25%), Clostridium perfringens type A (21% versus 10%), E. coli ag43 (20% versus 35%) were the most frequent enteric pathogens as detected in diarrheic and non diarrheic foals, respectively (Olivo et al., 2016). Additionally, an Indian study recorded that E. coli, K. pneumoniae, and P. mirabilis predominate in the sick and healthy foals (Singh, 2009).
The current results clarified the presence of co-infection which is more common than single infection, this observation was previously recorded by Slovis et al. (2014), who reported that the severity of the case becomes more worst in cases with more than one infectious agent. A single causative agent is often not found (Schoster et al., 2015), coinfection between infectious agents was recorded as being more prevalent and may result in more severe gastrointestinal disorders (Slovis et al., 2014).
The anaerobic cultivation of the examined samples in the present study revealed absence of Clostridium spp. in diarrheic foals, although previous studies reported that foal diarrhea under the age of one month usually attributed to the presence of Clostridium perfringens (Netherwood et al., 1996), while diarrheic foals above one month are usually due to rotavirus or Salmonella spp. (Frederick et al., 2009).
The absence of parasitic infection in the examined fecal samples was in contrast with previous results of Haq et al. (2017b) who showed that the common causative agents of diarrhea were helminths parasites (85%) by bacteria (55%). The dams are the most sources of parasitic infections to their offspring and administration of ivermectin to the dam can prevent the transmission of the parasitic infections to the foals (Mallicote et al., 2012). Our obtained results support this hypothesis as the case history revealed regular administration of anthelmintic drugs to the dams.
Although detection of one or more infectious agent from feces of an infected foal is not necessarily to be the responsible cause but isolation of the causative agents could be beneficial in designing the treatment plan (Frederick et al., 2009). Previous reports concluded that acute diarrhea often gives little time to establish the treatment, while a chronic disease keeps ongoing due to non-response to antimicrobial chemotherapy (Singh, 2009). Accordingly, antibiotic sensitivity test was carried out to detect the most effective antimicrobials. The obtained results showed that ciprofloxacin and gentamicin have a great effect in preventing the enterobacterial infections, followed by amikacin, neomycin, and rifamycin. Meanwhile, many isolated enteric bacteria showed drug resistance against ampicillin and erythromycin. Similar results were previously presented by (Singh, 2009) who recorded that all Gram positive and most of Gram negative bacteria had developed multiple-drug-resistance against some recent antibiotics like gatifloxacin, imipenem and ceftriaxone as it is recorded in bacteria isolated from feces of sick foals than the apparently healthy ones. Furthermore, he reported that all of the isolates were susceptible to gentamicin, although previous records declared that multiple and common use of this type in the equine veterinary clinics render many bacteria have raised resistance against it (Diab et al., 2013). Meanwhile, Orsini and Spencer (1997) reported that E. coli and Proteus spp. isolated from horses were susceptible to amikacin more than gentamicin. Concerning the variation in the susceptibility of the enterobacterial isolates against the antibacterial agents, Salmonella enteric was found to be susceptible to all the tested antibacterial agents in the current study. Moreover, Dadras et al. (1996) found that the highest percentage of E. coli and Proteus spp. isolates were resistant to ampicillin.
Hematological findings of diarrheic foals revealed markedly increased RBCs, and platelet counts and HCT level, while MCV and MCH values were significantly decreased compared to healthy ones. Microcytic normochromic anaemia was recorded in diarrheic foals caused by enterobacterial infections. Diarrhea is the main cause for hypovolemia (decreased plasma volume) due to dehydration resulted from water loss and/or splenic contraction which leads to increase the cellular constituents of blood (Southwood, 2013; Ijaz et al., 2019). The microcytic normochromic anaemia may be attributed to impaired duodenal mucosal uptake of the nutrients resulting in malabsorption and deficiency of vitamins and macro- and micro-minerals especially calcium, copper, and iron as it was previously recorded (Freeman, 2015; Ijaz et al., 2019). Thrombocytosis was recorded in 50% iron deficient patients. It may be due to elevation of erythropoietin or other cytokines (Thrall et al., 2012). In diarrheic foals, the total WBCs count was increased accompanied with increase in the lymphocytes count. These increases may be due to the occurrence of bacteremia (Weiss and Wardrop, 2010; Thrall et al., 2012).
In the serum of diarrheic foals, total proteins and albumin concentrations significantly decreased that may be due to malabsorption (most common in horses) and loss of intestinal mucosal integrity resulted from enterobacterial infections which develop protein losing enteropathy and subsequent hypoproteinemia (Jania and Andraszek, 2016). Unexpectedly, serum total proteins and albumin concentrations were significantly decreased. This is may be due to extravasation of plasma proteins which caused by enterocolitis affected the vasculature of intestinal villi (Magdesian et al., 2002). Regarding to serum protein electropherogram of Arabian horse foals, it is clearly separated into six different peaks (albumin, α1-, α2-, β1-, β2- and γ-globulin) which is consistent with the results of Gundasheva (2015). It was obvious from the current data that a significant increase in α2-globulin level was recorded in diarrheic foals. This increase may be attributed to the increased level of one or more of their main individual proteins such as α1-acid glycoprotein, serum amyloid A and P and haptoglobin which were confirmed to be elevated during infection and inflammation (Crisman et al., 2008; Soufy et al., 2017). Also, Kaneko et al. (2008) stated that increased α-globulin level in neonates and young animals is due to the increased concentrations of some proteins (e.g. α1-fetoprotein) which protect young animals from immunological attacks. Deficiency of β-globulins may be attributed to malnutrition (Tothova et al., 2016). On the other hand, the deficiency of γ-globulin may be ascribed to a failure in passive transfer of maternal immunoglobulins which increased the risk of infection (Fouché et al., 2014).
In diarrheic Arabian foals, serum urea was decreased which may be due to low protein intake resulted from malabsorption. Blood urea nitrogen is a metabolic end product of protein, synthesized in the liver and excreted by the kidneys. Serum urea concentration is determined by the balance between protein catabolism and renal excretory function. However, serum creatinine level and AST activity which were increased in diarrheic foals may be due to endotoxins of bacterial infection and elevation of AST activity also was attributed to muscle injury (Kaneko et al., 2008; Thrall et al., 2012).
In the present study, low serum calcium concentration in diarrheic foals reinforced with the earlier data in which calcium concentration decreased in colic horse with diarrhea (Navarro et al., 2005), horse with gastrointestinal disease, and diarrheic foals (Ijaz et al., 2019). Hypocalcemia might be due to modifications in blood pH and albumin level, infection, food deficiency, and intravenous fluid therapy without calcium supplementation (Navarro et al., 2005). There was a non-significant change in serum inorganic phosphorus level.
Electrolytes imbalance is one of the most common features in diarrhea. Hyponatremia in diarrheic foal was noticed and coincided with the earlier findings (Magdesian et al., 2002; Gomez et al., 2013; Ijaz et al., 2019). Hyponatremia may be attributed to gastrointestinal loss (vomiting, diarrhea), renal losses (hypoadrenocorticism, prolonged diuresis) and sweating. Sodium-containing fluid losses in diarrhea are the main cause for decreased plasma volume. As plasma volume decreased, the HCT increased (Kaneko et al., 2008). This is in accordance with the present data. The non-significant decrease in serum potassium concentration was recorded in the present data. This finding disagrees with the previous reports which showed hypokalemia (Gomez et al., 2013) and hyperkalemia (Ijaz et al., 2019) in foal suffering from diarrhea.
CONCLUSION
The major causative agents of diarrhea were Enterobacter aerogens followed by Proteus mirabilis, E. coli, Citrobacter diversus, Salmonella enterica and Proteus vulgaris. The most effective antibacterial agents were ciprofloxacin and gentamicin followed by amikacin, neomycin, and rifamycin against Gram negative enterobacterial species isolated from diarrheic foals. Antibiotic susceptibility can help the choosing the effective antibiotic for therapy. In addition, hematological, metabolic and electrolytes disturbances (microcytic normochromic anaemia, leukocytosis and lymphocytosis, hyporoteinaemia, hypoalbuminaemia, hypoglobulinaemia (β2- and γ-globulins), decreased serum urea, calcium and sodium, and increased serum α2-globulin, AST and creatinine) were recorded in darrhoeic Arabian horse foals caused by enterobacterial infections.
AUTHORS CONTRIBUTION
All authors shared equally in designing, conducting the study and writing the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
REFERENCES





