Advances in Animal and Veterinary Sciences
Short Communication
Synthesis of (Hydroxylapatite: Silver) Mixing with Coaltar for Psoriasis Therapy
Asmaa Hadi Mohammed1, Marwa Abdul Muhsien Hassan1*, Majid Shanon Khalaf2
1Department of Physics, College of Science, Mustansyriah University, Baghdad, 00964, Iraq; 2Ministry of Science and Technology, Department of Nuclear, Biological and Military Waste, Iraq.
Abstract | Psoriasis is a critical and serious disease which can affect the immunity system of the body resulting in a rapid buildup of skin cells. Interests come over this research to know the role of nanomaterial in Psoriasis treatment, so here, Nano biological-materials were prepared from different natural resources for both Hydroxyapatite and Silver using a simple chemical method. The nano products have been characterized using various techniques to measure the structural properties and the grain size. To manifest the appropriate preparation conditions for treating psoriasis, the nano product has been mixed with the coaltar.
Keywords | Hydroxylapatite, Ag, Coal tar and psoriasis treatment
Received | September 28, 2019; Accepted | March 03, 2020; Published | March 20, 2020
*Correspondence | Marwa Abdul Muhsien Hassan, Department of Physics, College of Science, Mustansyriah University, Baghdad, 00964, Iraq; Email: marwamedicalphysics@gmail.com
Citation | Mohammed AH, Hassan MAM, Khalaf MS (2020). Synthesis of (Hydroxylapatite: Silver) mixing with coaltar for psoriasis therapy. Adv. Anim. Vet. Sci. 8(4): 360-363.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.360.363
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Mohammed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Psoriasis is a common skin disease that affects the life cycle of skin cells (Jones et al., 2003; Jump, 2013; Menter et al., 2008; Ely and Seabury, 2010; Boehncke and Schön, 2015). As a result of psoriasis, cells quickly build up on the surface of the skin to form thick silver scales and itchy, dry and red layers, sometimes causing pain (Jain, 2012; Parisi et al., 2013; Tamparo, 2011). Psoriasis is a stubborn disease that lasts for a long time (chronic disease). There are periods in which the symptoms of psoriasis improve and alleviate, while psoriasis intensifies in other periods (Palfreeman et al., 2013; Colledge et al., 2010; Zattra et al., 2012; Stanway, 2014; James et al., 2005; Robinson et al., 2012). For some patients, psoriasis is a nuisance. For others, it is possible to cause disability, especially when they are associated with arthritis (Arthritis). Psoriasis is not cured, but psoriasis treatment can significantly improve (Raychaudhuri et al., 2014; Weigle and McBane, 2013; Gudjonsson et al., 2012; Gelmetti, 2009; Yesudian et al., 2012; Ship et al., 2008). Lifestyle measures, such as the use of cortisone ointment, moderate skin exposure to the sun in a controlled manner, can improve psoriasis symptoms.
Materials and Methods
The method of co-precipitation was used to synthesize hydroxyapatite (HAp) crystals. A solution of 0.6 M H3PO4 under continuous stirring at room temperature was added to a 1 M Ca(OH)2 and 0.5 M Ag(NO3)2 aqueous solution. By adding an ammonia solution, the final pH was adjusted to 11. The resulting precipitate was aged in stirring for 24 hours. Centrifugation collected the obtained white precipitates and washed repeatedly with distilled water. The product was dried for 24 hours in an oven at 80 oC. The obtained HAp crystals were ground with a mortar and pestle, then they were sieved at 45 μm. Finally, in a conventional furnace, the HAp crystals were calcinated for 2 hours in an air atmosphere at 1200 oC. Then the HAp: Ag mixing 50:50 percent with coaltar.
Results and Discussion
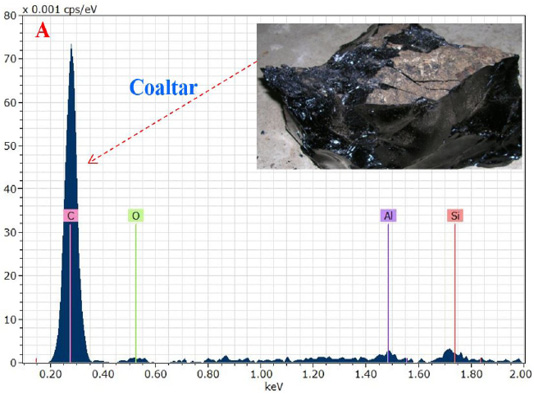
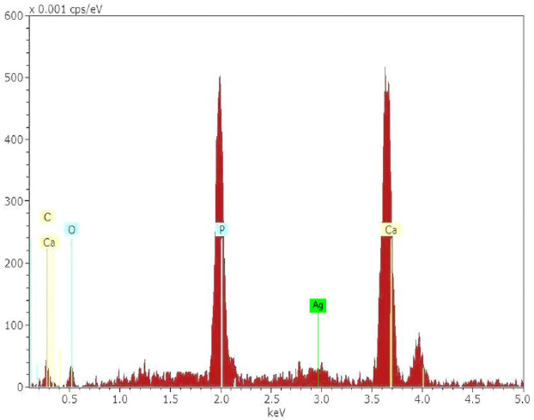
The chemical analysis results showed that the stone coaltar is a very pure containing an element (C, O, Al and Si) as shown in Figure 1A. Where the proportion of carbon element was the highest value in the coaltar and this confirms the purity of the material used for treatment after mixing with the prepared hydroxyapatite: Ag nanomaterial. The result of the energy-dispersive X-ray spectroscopy (EDXS) showed that only Ca, P, Ag and O were composed as synthesized HAp: Ag nanorods. The energy-dispersive X-ray spectroscopy (EDXS) detects no other peaks related to any impurity. Semiquantitative analysis shows that the Ca/P molar ratio is approximately 1.67 and closer to the theoretical value of 1.67, confirming that the nanorods are hydroxyapatite treated at 1200 °C as shown in Figure 1B. The C-related peak comes from the FESEM substratum.The results of the FESEM-EDXS analysis (Figure 1) showed that the nano-pure and doped HAp Ca / P ratio investigated in this study varies with an average of 1.67 between 1.46 and 2.01. The main difference between synthetic and natural hydroxyapatite is that the synthetic material showed a higher Ca/P ratio. While the former was closer to the hydroxyapatite stoichiometric. It should be noted that the semi-quantitative analysis of the Ca/P ratio of hydroxyapatite using SEM-EDXS has a technical problem. In other words, the X-ray intensity of phosphorus decreases faster than that of calcium during the analysis at a fixed current density and increasing irradiation time, resulting in an increase in the Ca / P ratio at a high dose of electron irradiation. Thus, the accelerating voltage was set at 15 kV in this study and the analysis was carried out at a low irradiation time to overcome the above problem.
Figure 2 shows SEM images of 600 oC, 800 oC, 900 oC and 1200 oC heat-treated samples respectively. The powder obtained at temperatures between 600 oC and 800 oC after heat-treatment consists of well-defined, randomly oriented elongated nanorods. As the temperature raises to 900 oC, elongated nanorod shapes are maintained, but sphere-like structures are observed for temperatures above 900 oC. Figure 2 shows the mean nanorod diameter value versus the temperature of the heat treatment. The average diameter of the nanorods takes values in a rather narrow interval for temperatures between 600 oC and 800 oC, which increases with increasing temperature. Although the average diameter shows a slight decrease at 900 oC, this is not a major problem. On the other hand, the mean diameter increases sharply for sintering temperatures above 900 oC as the temperature rises, whereby the diameter values are spreading larger. The large spread may be due, as can be observed in Figure 2, to the partial or incipient fusion of adjacent nanorod surfaces. The length of nanorods ranges from 100 nm to about 200 nm, but no correlation could be established between the length and the sintering temperature.
Treatment of psoriasis using (Hydroxyapatite: Silver) mixing with coaltar
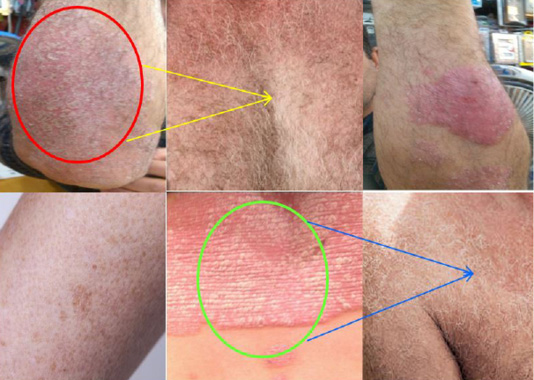
Psoriasis is caused by a cause related to the immune system, specifically a specific type of white blood cell called (T cell) lymphocytes as shown in Figure 3. Normally, these cells travel throughout the body to find and control foreign substances, such as bacteria and viruses. But in psoriasis patients, these lymphocytes accidentally attack healthy skin cells. Too effective T lymphocytes provoke various reactions in the immune system, such as enlargement of blood vessels around the skin layers and increasing amounts of other blood cells that can penetrate the epidermis (thin outer layer of skin-Epidermis). As a result of these changes, the body produces more healthy skin cells, more T lymphocytes and other white blood cells. As a result, new skin cells reach the outer layer of skin very quickly in a few days, rather than weeks as is normal. But dead skin cells and white blood cells cannot rapidly fall off, so they accumulate in thick crust layers on the surface of the skin. This process can be stopped, mostly, by treatment. It is not clear exactly why T-lymphocyte activity is disrupted in psoriasis patients, and researchers believe that both genetic and environmental factors play a role.
The desired goals of psoriasis treatment using (hydroxyapatite: Ag nanoparticles mixing with coal tar)
- 1. Stop the process that leads to the production of excess skin cells, which reduces inflammation and the formation of layers.
- 2. Remove the dandruff and make the skin soft.
Conclusions
The use of a chemical method in the preparation of biological material in the form of nano-rod and mixing with coal tar and used to eliminate psoriasis in humans.
Authors Contribution
All authors contributed equally.
Conflict of interest
1- Psoriasis has increased worldwide.
2- Difficulty treating psoriasis once and for all using traditional treatments.
3- The possibility of using nanomaterials to improve the action of coal tar for the ultimate treatment of psoriasis.
REFRENCES