Advances in Animal and Veterinary Sciences
Research Article
Antibiotic Resistance in Escherichia coli Isolated from Döner Kebab Sold in Dramaga Bogor, Indonesia
Devi Yanti Sari1*, Herwin Pisestyani2, Denny Widaya Lukman2
¹Graduate School, Veterinary Public Health Study Program, IPB University, Indonesia; ²Department of Animal Infectious Diseases and Veterinary Public Health, Faculty of Veterinary Medicine, IPB University, West Java, Indonesia 16680.
Abstract | This study was aimed to determine the occurrence of antibiotic resistance in Escherichia coli (E. coli) isolated from Döner kebab sold in Dramaga Bogor, West Java Province, Indonesia. A total of 43 samples of Dὂner kebab meat were taken from all of the vendors in the radius of 2 km around the border of IPB University campus, Dramaga Bogor. Isolation and identification of E. coli were referred to the Guideline for Laboratory Analysis on Examination of Microbial Contamination in Meat, Egg, and Milk in SNI 2897:2008 issued by the National Standardization Agency of Indonesia. The resistance against nine antibiotics were tested using Kirby-Bauer disk diffusion method based on the standard of Clinical Laboratory Standards Institute (CLSI) in 2018. The result showed that nine samples were positive containing E. coli (20.9%) and 84.2% of E. coli isolates were resistant to eight antibiotics: gentamicin (57.9%), ampicillin (26.3%), cefotaxime, trimethoprim-sulfamethoxazole, and ciprofloxacin (21.1%), amoxicillin-clavulanate (10.5%), enrofloxacin, and oxytetracycline (5.3%). All of E. coli isolates were susceptible to chloramphenicol (100%). There were 21.1% of isolates also resistant to three or more classes of antibiotics that were known as multi-drug resistant (MDR) with different resistant patterns.The presence of E. coli resistant antibiotic in kebab meat could results serious problems in human health.
Keywords | Antibiotic resistance, AMR, E. coli, MDR, Döner kebab
Received | November 26, 2019; Accepted | February 26, 2020; Published | March 03, 2020
*Correspondence | Devi Yanti Sari, Graduate School, Veterinary Public Health Study Program, IPB University, Indonesia; Email: devi.y.sari@gmail.com
Citation | Sari DY, Pisestyani H, Lukman DW (2020). Antibiotic resistance in Escherichia coli isolated from döner kebab sold in Dramaga Bogor, Indonesia. Adv. Anim. Vet. Sci. 8(3): 278-284.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.3.278.284
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Antimicrobial resistance has become a significant public health problem in the world. It was estimated that in 2050 antimicrobial resistance could cause 50 million deaths per year all over the world (O’Neill, 2014). World Health Organization (WHO) defines antimicrobial resistance (AMR) as the ability of a microorganism to stop antimicrobial agents from working against it. Antimicrobial resistance can cause ineffectiveness in standards treatment, persistent infections, and may spread to others (WHO, 2019). Inappropiate antimicrobial usage in livestock, aquaculture, pets, crops, and humans caused antimicrobial-resistant bacteria that settle in agricultural and clinical biomes. Antimicrobial resistance in pathogen resulting from extensive and uncontrolled antimicrobial agents in agricultural and medicine (Cameron and McAllister, 2016).
The application of antibiotics in food-producing animals has become significant concern in food safety. It is because foods of animal origin are sometimes identified as the vehicles of foodborne disease in humans and can act as vehicles of resistant foodborne pathogens and resistant genetic material (Malathi et al., 2014). Commensal bacteria and pathogen bacteria like Escherichia coli (E. coli) can develop resistance to antibiotics. Pathogenic Escherichia coli can cause foodborne disease because of the consumption of contaminated or undercooked meat (Frye et al., 2013). Escherichia coli was one of the main microorganisms that cause antimicrobial resistance (WHO, 2017).
The kebab was one of the Middle East’s ready-to-eat foods and has become famous around the world, including in Indonesia. Döner kebab was one of the kebab variances that generally sold in Indonesia, especially in urban areas. Döner kebab prepared by marinating meat or minced meat, placed on the rotisserie, and roasted in a vertically positioned cooker. During cooking, the cooked part is cut away with a long-bladed or circular knife and stuffed into pita bread along with sliced tomatoes, onions, and lettuce (Kaya et al., 2018; Liuzzo et al., 2016). There are some issues related to microbiological quality and formulation in döner kebab. Using low-quality raw meat and under cooked meat may cause food safety issues in kebab (Ergonul et al., 2012). Escherichia coli was one of the bacteria that was related to foodborne disease in kebab (Bonilauri et al., 2018).
Escherichia coli can be found in the environment, food, animals, and human intestines. Escherichia coli can act as commensal and pathogen bacteria (CDC, 2019; Rivas et al., 2019). Escherichia coli was Gram-negative bacteria, belongs to Enterobacteriaceae family, rod-shaped, size 2.0-6.0 μm (length), and 1.1 -1.5 μm (width) (Percival and Williams, 2014). Escherichia coli can cause extraintestinal infection, enteric disease, and systemic infection in humans and animals (Ramirez-Castillo et al., 2018). Escherichia coli O157:H7 was one of the E. coli pathogen strain that caused severe disease like hemolytic uremic syndrome in humans (Rivas et al., 2019).
Kebab contaminated by resistant pathogen bacteria can cause severe illness. Antibiotic-resistant bacteria, including E. coli in animals, can act as a reservoir infection in humans (Sahoo et al., 2012). Students of IPB University stay around the campus and mostly in the radius of 2 km (kilometre) around the border of the campus of IPB University. Most of the students eat in the canteen or buy the RTE foods that are sold around the campus. Döner kebab is one of the favorite food for the students. There is lack of study on the occurance of E. coli and the antibiotic-resistance in Döner kebab meat. Therefore, this research was conducted to observe the occurance of antibiotic-resistant E. coli in Döner kebab sold at Dramaga Bogor.
MATERIAL AND METHODS
Material
Material used in this study were kebab meat, 0.1% buffered peptone water (BPW) (Oxoid CM1049, England), lauryl tryptose broth/lauryl sulphate broth (LTB) (Oxoid CM0451, England), EC (Escherichia coli) broth (Oxoid CM0853, England), eosin methylene blue agar (Levine) (L-EMB) (Oxoid CM0069, England), methyl red-Voges Proskauer (MR-VP) medium (Oxoid CM0043, England), Sim medium (Oxoid CM0435, England), Koser citrate broth (Conda Cat. 1200.00, Spain), nutrient agar (NA) (Oxoid CM0003, England), Kovacs indole reagent (Merck 1.09293.0100, Germany), KOH 40%, Mueller Hinton agar (MHA) (Oxoid CM0337, England), tryptone soya broth (TSB) (Oxoid CM0129, England), E. coli American Type Culture Collection (ATCC) 25922 (Thermo), antibiotic disk, i.e., ampicillin (AMP) 10 μg (Oxoid AMP 10 CT0003B, England), amoxicillin-clavulanate (AMC) 30 μg (Oxoid AMC 30 CT0223B, England), cefotaxime (CTX) 30 μg (Oxoid CTX 30 CT0166B, England), gentamicin (CN) 10 μg (Oxoid CN 10 CT0024B, England), trimethoprim-sulfamethoxazole (SXT) 25 μg (Oxoid SXT 25 CT0052B, England), ciprofloxacin (CIP) 5 μg (Oxoid CIP 5 CT0425B, England), enrofloxacin (ENR) 5 μg (Oxoid ENR 5 CT0639B, England), oxytetracycline (OT) 30 μg (Oxoid OT 30 CT0041B, England), and chloramphenicol (CL) 30 μg (Oxoid CL 30 CT0013B, England).
Collection of samples
Döner kebab meat samples (25 grams) were collected from all of the vendors in the radius of 2 km around the campus of IPB University, Dramaga Bogor, where most of the students stayed in that area. There were 9 vendors which sold Döner kebab. In every vendor was taken five samples in five different days. Total samples of kebab meat collected were 43 samples. Sample collected in the evening or a busy hour at 7.00-9.00 p.m. where the number of customers reached its peak. The obtained samples were inserted into sterile plastic bags and labelled with the identity of the sample, then stored in a cool box with a temperature of 4-10 ºC and immediately taken to the Laboratory of Veterinary Public Health, Faculty of Veterinary Medicine, IPB University for isolation test and identification of E. coli. The antimicrobial resistance test was also conducted against nine selected antibiotics (ampicillin, amoxicillin-clavulanate, cefotaxime, gentamicin, trimethoprim-sulfamethoxazole, ciprofloxacin, enrofloxacin, oxytetracycline, and chloramphenicol).
Isolation and identification of Escherichia coli
Isolation and identification test of E. coli were referred to the standards of the National Standardization Agency of Indonesia (BSN) according to the Guideline for Laboratory Analysis on an Examination of Microbial Contamination in Meat, Egg, and Milk in SNI 2897:2008. The procedure also followed the Compendium of Methods for The Microbiological Examination of Foods 2001 (Downes and Ito, 2001). Quality control of each test were conducted using E. coli American Type Culture Collection (ATCC) 25922 (Thermo). Most Probable Number (MPN) method three tubes dilution was used in this study.
Twenty-five grams of Döner kebab meat was added into 225 ml 0.1% buffered peptone water (BPW) (Oxoid CM1049, England) and homogenized with stomacher for 1 minute. The homogenized samples were then transferred into lauryl tryptose broth/lauryl sulphate broth (LTB) broths (Oxoid CM0451, England), and incubated at 35 ºC for 24-48 hours for MPN presumptive test. The positive results, which were shown as gas formed in the Durham tube then transferred into E. coli (EC) broth (Oxoid CM0853, England) and incubated at 45.5 ºC for 48 hours for MPN-confirmed test. Positive results in EC broth then inoculated to selective medium Levine Eosin Methylene Blue Agar (L EMBA) (Oxoid CM0069, England) and incubated at 35 ºC for 18-24 hour. The expected E. coli colony were shown as black/dark color, while the center of the colony was metallic green. Suspicious colonies from each L-EMB plate inoculated to NA slant and incubated for 18-24 hours at 35ºC for further test. The biochemical test (five essential sugars) was carried out for confirmation consisting of indole, methyl red, Voges-Proskauer, and citrate (IMViC). All cultures that give IMViC patterns of ++-- or -+-- were considered to be E. coli.
Antibiotic resistance test
Antibiotic resistance test was conducted to all E. coli colonies isolated from kebab samples and referred to standard on Clinical Laboratory Standard Institute (CLSI, 2018) using the Kirby-Bauer disk diffusion method. The antibiotics resistance test were conducted against nine kinds of antibiotics, i.e., ampicillin (AMP) 10 μg (Oxoid AMP 10 CT0003B, England), amoxicillin-clavulanate (AMC) 30 μg (Oxoid, AMC 30 CT0223B, England), cefotaxime (CTX) 30 μg (Oxoid CTX 30 CT0166B, England), gentamicin (CN) 10 μg (Oxoid CN 10 CT0024B, England), trimethoprim-sulfamethoxazole (SXT) 25 μg (Oxoid SXT 25 CT0052B, England), ciprofloxacin (CIP) 5 μg (Oxoid CIP 5 CT0425B, England), enrofloxacin (ENR) 5 μg (Oxoid ENR 5 CT0639B, England), oxytetracycline (OT) 30 μg (Oxoid OT 30 CT0041B, England), and chloramphenicol (CL) 30 μg (Oxoid CL 30 CT0013B, England). Suspension of bacteria equivalent to 0.5 McFarland turbidity standard (1-2x108 CFU/mL) was prepared. The culture was taken using a sterile cotton swab, spread on Mueller Hinton Agar (MHA) (Oxoid CM0337, England England) surface, and left for ± 5 minutes. The paper disk contained an antibiotic then was put on MHA, which was previously spread by the pure culture at a distance of 25-30 mm. The cultured bacteria were incubated at 35 ºC for 18-24 hours. Categories of susceptible, intermediate, and resistant were based on the size of the inhibition zone formed according to the standard of CLSI, (2018). A blank disk without antibiotic was used as a negative control for each test.
Data analysis
Data were analyzed descriptively in the form of figures and tables.
RESULTS
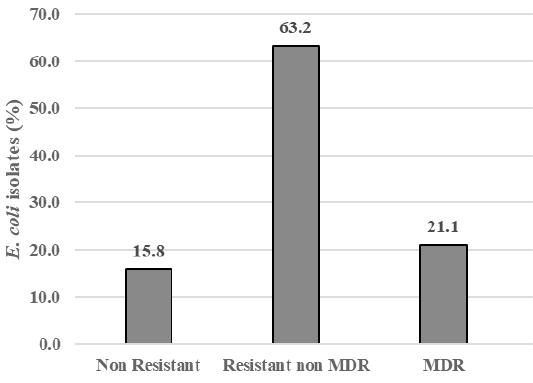
The result showed that among 43 samples of Döner kebab meat, nine samples were positive containing E. coli (20.9%). There were 19 E. coli isolates,and 84.2% (16/19) isolates were resistant to antibiotics, i.e., gentamicin (57.9%), ampicillin (26.3%), cefotaxime, trimethoprim-sulfamethoxazole, and ciprofloxacin (21.1%), amoxicillin-clavulanate (10.5%), enrofloxacin, and oxytetracycline (5.3%). All isolates were susceptible to chloramphenicol (100%) (Figure 1). There were 15.8% isolate was sensitive to antibiotics (Non Resistant), 84.2% resistant to antibiotics that included 63.2% resistant to at least two classes of antibiotics (Resistant non MDR), and 21.1% of isolates (4/19) also resistant to three or more classes of antibiotics or known as multi-drug resistant (MDR) (Figure 2). The resistance pattern was shown: cefotaxime-gentamicin-ciprofloxacine (CTX-CN-CIP), ampicillin-cefotaxime-trimethoprim sulfamethoxazole-gentamicin (AMP-CTX-SXT-CN), ampicillin-enrofloxacin-ciprofloxacin-gentamicin (AMP-ENR-CIP-CN), ampicillin-cefotaxime-trimethoprim sulfamethoxazole-oxytetracycline-gentamicin-ciprofloxacin (AMP-CTX-SXT-OT-CN-CIP) (Table 1).
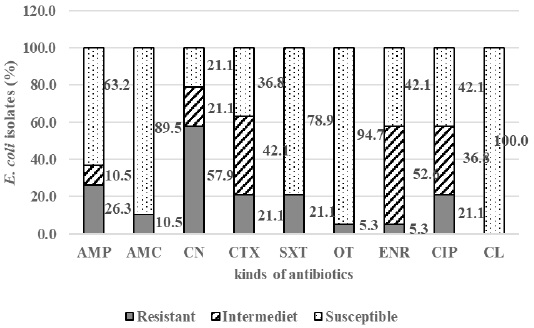
Figure 1: The percentage of E. coli susceptible, intermediate, and resistant in Döner kebab meat sold at Dramaga Bogor; ampicillin (AMP), amoxicillin-clavulanate (AMC), enrofloxacin (ENR), cefotaxime (CTX), trimethoprim-sulfamethoxazole (SXT), oxytetracycline (OT), gentamicin (CN), ciprofloxacin (CIP), and chloramphenicol (CL).
DISCUSSION
Based on this study, there was a high percentage of positive E. coli in Döner kebab meat samples (20.9%). The results were similar to the study conducted by Amani et al. (2017) that showed 33.33% of shawarma beef contaminated by E. coli in Ashmoun, Menofia Egypt. The high percentage of
Table 1: Multi-drug resistant (MDR) and resistant pattern of E. coli in Döner kebab meat sold at Dramaga Bogor.
| Isolates | % isolates | Resistant pattern | |
| Non Resistant (susceptible) | 3 | 15.8 | |
| Resistant not MDR | 12 | 63.2 | |
| MDR: | |||
| Resistant to 3 classes antibiotics | 2 | 10.5 | CTX-CN-CIP, AMP-ENR-CIP-CN |
| Resistant to 4 classes antibiotics | 1 | 5.3 | AMP-CTX-SXT-CN |
| Resistant to 6 classes antibiotics | 1 | 5.3 | AMP-CTX-SXT-OT-CN-CIP |
AMP: ampicillin; AMC: amoxicillin-clavulanate; ENR: enrofloxacin; CTX: cefotaxime; SXT: trimethoprim-sulfamethoxazole; OT: oxytetracycline; CN: gentamicin; CIP: ciprofloxacin; CL: Chloramphenicol.
kebab that contaminated by E. coli from this studywas alarming because E. coli pathogen could cause foodborne disease to the consumers. Since the Döner kebab was mostly consumed by the students, it might cause the foodborne disease in the students.
The use of low-quality meat and poor hygiene practices in kebab vendors around Dramaga Bogor in this study could be contributed to the prevalence of E. coli in this study. According to Ergonul et al. (2012), the use of low-quality raw meat and undercooked kebab meat could cause food safety issues. Poor handling practices also significantly contributed to E. coli contamination (Kwiri et al., 2014). It has been reported that hygiene indicator organisms (E. coli) were most often detected in meat preparations. The source of microorganisms that contaminated meat were spices and other ingredients, as well as from processing environment, types of equipment, and handlers that could have a significant impact on the microbiological status of the end-products (Melngaile et al., 2014).
The result of antibiotic resistance test on E. coli isolates showed that among 19 isolates there were 84.2% isolates were resistant to eight kinds of antibiotics, i.e., gentamicin (57.9%), ampicillin (26.3%), cefotaxime, trimethoprim-sulfamethoxazole, and ciprofloxacin (21.1%), amoxicillin-clavulanate (10.5%), enrofloxacin, and oxytetracycline (5.3%). All isolates were susceptible to chloramphenicol (100%) (Figure 1).
Contamination of E. coli resistant bacteria can occur from slaughterhouse to consumers table. Recent study showed that E. coli was isolated from the environment of Bogor Slaughterhouse, Indonesia and resistant to penicillin G (100.0%), streptomycin (100.0%), followed by gentamicin (60.0%), trimethoprim-sulfamethoxazole (60.0%), tetracycline (40.0%), ciprofloxacin (40.0%), enrofloxacin (20.0%) (Sudarwanto et al., 2017).
The highest antibiotics resistance in E. coli isolated from kebab meat was against gentamicin (57.9%). The results were similar to the study conducted by Badi et al. (2017), which showed that E. coli resistance to gentamicin was high (75.3%). However, Rahman et al. (2017) reported that gentamicin was still susceptible (100%). The high resistance of gentamicin in E. coli isolates was uncommon found in animals (Kaesbohrer et al., 2012). Meanwhile Tadesse et al. (2012) reported that gentamicin resistance in E. coli isolates from animals has been increasing. Gentamicin was widely used in the poultry industry, and antibiotics in aminoglycoside groups were used in food animals. The resistance to gentamicin has been reported slightly higher in poultry and cows than in pigs.
The E. coli resistance to trimethoprim-sulfamethoxazole in this study were 21.1%, that was similar to the research conducted by Badi et al. (2018) which showed that E. coli resistance to trimethoprim-sulfamethoxazole were 36.9%. The second-highest resistance in this study occurred to ampicillin (26.3%), contrary to the study conducted by Messele et al. (2017) that found high occurance (71.4%) of the E. coli resistance to oxytetracycline (5.3%) and ciprofloxacin (21.1%) in this study was lower than the study conducted by Rahman et al. (2017) which reported the resistance in oxytetracycline (71.43%) but not resistant to ciprofloxacin (100% sensitive).
The resistance to cefotaxime in this study (21.1%) was higher compared to the study conducted by Badi et al. (2018) which found a 7.7% of resistance in cefotaxime. The resistance in cefotaxime was considered as an early warning because cefotaxime was one of the third generations of cephalosporin that was known as drugs of choice in bacteremia caused by Gram-negative bacteria in humans (Park, 2014). Durmaz et al. (2015) reported that E. coli was resistant to many cephalosporins, fluoroquinolones, and other first-generation antibiotics. Wang et al. (2015) reported the increase of E. coli resistance to ciprofloxacin and third generation of cephalosporins, especially cefotaxime in E. coli.
All of the isolates showed susceptible (100%) to chloramphenicol, contrary to the study conducted by Beyi et al. (2017) that reported the resistance of E. coli to chloramphenicol. Chloramphenicol is bacteriostatic for Gram-positive bacteria and Gram-negative bacteria. These antibiotics are prohibited from being used in food animals in many countries because it was related to idiosyncratic fatal aplastic anemia in human (Maddison et al., 2008). The use of chloramphenicol in food animals has been also prohibited in Indonesia (Kementan, 2017). Chloramphenicol residues can persist for extended periods in food animals and residues in animal products can cause public health risk (Papich, 2018).
As seen in Figure 2, there were 15.8% isolates which were sensitive to antibiotics and 84.2% isolates which were resistant to antibiotics. From antibiotic-resistant E. coli isolates, 21.1% (4/19) isolates showed resistance to three or more classes of antibiotics or known as multi-drug resistant (MDR). This finding was similar to the study of Messele et al. (2017) which reported MDR in raw meat isolates were 46%. The resistance pattern in this study showed the variance pattern, i.e., cefotaxime-gentamicin-ciprofloxacine (CTX-CN-CIP), ampicillin-cefotaxime-trimethoprim sulfamethoxazole-gentamicin (AMP-CTX-SXT-CN), ampicillin-enrofloxacin-ciprofloxacin-gentamicin (AMP-ENR-CIP-CN), ampicillin-cefotaxime-trimethoprim sulfamethoxazole-oxytetracycline-gentamicin-ciprofloxacin (AMP-CTX-SXT-OT-CN-CIP) (Table 1).
The previous study reported E. coli (80%) isolated from the environment of Bogor slaughterhouse were resistant to at least three antibiotic classes. The spread of these organisms could be a problem of food safety, human, animals, and other pathogen bacteria (Sudarwanto et al., 2017). The increasing occurrence of MDR in E. coli in human and veterinary medicine has become an alarming issue (Poirel et al., 2018). Multi-drug resistance can increase failure and frequency on medication (Ramírez-Castillo et al., 2018).
CONCLUSION
The food safety level in Döner kebab sold in Dramaga Bogor was critical because there were 20.9% of E. coli positive samples. The occurrence of E. coli resistant antibiotics was high, in which 84.2% of the E. coli isolates were resistant to nine kinds of antibiotics, and multi-drug resistance was also found among isolates (21.1%). These results can lead to public health problem, i.e., foodborne disease and antibiotic resistance in the future.
ACKNOWLEDGMENT
This research was supported by the scholarship from The Ministry of Agriculture, Indonesia.
AUTHORS CONTRIBUTION
The research was designed jointly by Devi Yanti Sari, Herwin Pisestyani, Denny Widaya Lukman. Devi Yanti Sari conducted the research. The authors read and approved the final manuscript.
Conflict of interest
There is no conflict of interest in this article to declare.
REFERENCES