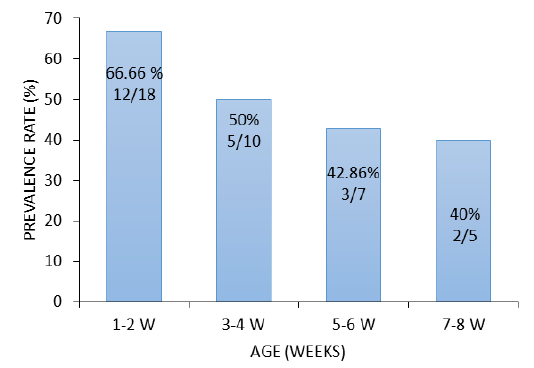Advances in Animal and Veterinary Sciences
Research Article
Prevealnce and Molecular Characterization of Avian Pathogenic E. Coli in Ducks
Lammah Kamel Abd El-Samie1*, Amany M. Abd-Elmoaty2, Nermin A. Ibrahim2, Wageh Sobhy Darwish3
1Avian and Rabbit diseases, The Educational Veterinary Hospital, Zagazig University 44519, Egypt; 2Department of Bacteriology, Mycology and Immunology, Faculty of Veterinary Medicine, Mansoura University, Egypt; 3Department of Food Control, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt.
Abstract | Avian pathogenic E. coli (APEC) represents a major threat of the Egyptian poultry industry in and worldwide leading to high economic losses. This study aims to investigate the prevalence of APEC strains and its tissue distribution in diseased ducks of different ages in Sharkia governorate, Egypt, between June and September 2018. Detection of virulence-associated genes among the identified serotypes was carried out using multiplex PCR. Also, the antimicrobial susceptibility of the isolated serotypes was examined using the disk-diffusion method. Our results revealed that E. coli isolation rate was 69 out of 200 samples (34.5%) and the highest prevalence was recorded at ducklings of 1-2 weeks old. Liver had the highest prevalence rate among the examined tissue (50%), followed by spleen (42.5%), heart (30%), lungs (25%) and gizzards (25%), respectively. Six serotypes were successively identified; E. coli O55:H7, E. coli O127:H6, E. coli O111:H4, E. coli O114:H21, E. coli O78:H- and E. coli O26:H11 at which E. coli O55:H7 serotype was the highest isolation rate 27.54% (19/69). Such pathogenic serotypes harbored virulence-associated genes including tsh, astA and iroN. Finally, antimicrobial sensitivity testing indicated that the isolated E. coli serotypes had multidrug-resistant profiles while gentamycin, neomycin and lincomycin are considered as promising candidates for the control of E. coli infection in ducks in Egypt.
Keywords | E. coli, Ducks, Virulence genes, Antimicrobial sensitivity test
Received | September 19, 2019; Accepted | October 26, 2019; Published | September 12, 2019
*Correspondence | Lammah Kamel Abd EL-Samie, Avian and Rabbit diseases, The Educational Veterinary Hospital, Zagazig University 44519, Egypt; Email: lamahsamie@gmail.com
Citation | EL-Samie LKA, Abd-Elmoaty AM, Ibrahim NA, Darwish WS (2019). Prevealnce and molecular characterization of avian pathogenic E. coli in ducks Adv. Anim. Vet. Sci. 7(s2): 163-168.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.163.168
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 El-Samie et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Escherichia coli (E. coli) is a major threat affecting poultry industry in Egypt and worldwide. E. coli is normally inhabited in the intestinal tract of birds. However, E. coli O26, O78, O114 and O127 serotypes have acquired some virulence-associated genes that enabled them to cause intestinal diarrhea and hemorrhage (Darwish et al., 2015). Such serotypes are known as avian pathogenic E. coli (APEC) (Ruttler et al., 2006; Xia et al., 2010). APEC may cause colisepticemia, yolk sac infection, cellulitis, coligranuloma and omphalitis in ducks and other bird species (Salehi and Ghanbarpour, 2010). However, there is a lack of information about both the incidence and virulence-associated genes characterization for the extra-intestinal E. coli infections in ducks at Egypt.
Darwish et al. (2013) mentioned that antibiotics such as amoxicillin is regularly used in hen farms for prevention and control of bacterial diseases in Egypt. Moreover, several classes of the antimicrobials are used as feed additives to stimulate the growth and improve bird’s feed conversion ratio. However, the extensive and abuse of such antimicrobials may lead to development of drug resistant bacterial strains. Therefore, it is of value to monitor the antibiogram of such pathogenic organisms.
Ying et al. (2010) used PCR assay for detection of APEC isolates and isolates from healthy ducks for characterization colonization factors F1-fimbriae (fimC), resistance serum survival protein (iss), invasion of brain endothelium protein A (ibeA), outer membrane protein A (ompA), the iron acquisition-related factors ferric yersiniabactin (fyuA), iron repressible protein (irp2), haemolysin A and E (hlyA and hlyE), Shiga toxin 1 and 2 (stx 1 and stx 2), intimin (eaeA), transcription antiterminator (rfaH), as well as betA gene (encoding choline dehydrogenase).
Moreover, Nokukhanya and Joshua (2018) have been studied E coli virulence genotyping from 45 different birds by using four different multiplex PCRs to investigate the presence of 12 virulence genes associated with APEC. Ewers et al. (2009) noted that virulence genotyping was considered to be the best way for differentiation between APEC and avian fecal E coli (AFEC), while serotyping might not be particularly useful due to the overlap in serogroups not only between APEC and AFEC but also between APEC and other extra-intestinal pathogenic E coli (ExPEC) isolates.
The present study aims to study the prevalence of the APEC strains in diseased duck flocks in Sharkia governorate, Egypt. Detection of the virulence-associated genes among the identified serotypes was conducted using PCR followed by the antibiogram examination using the disk-diffusion method.
Materials and Methods
Specimens
Two hundred specimens were collected from heart, lungs, liver, spleen and gizzards (40 each) from 40 ducks (morbid or freshly dead) between June and September 2018. The age of ducks ranged between one to eight weeks (W) with history of anorexia, poor growth associated with diarrhea. In some cases, respiratory symptoms were observed in some cases. The specimens were transported without delay in a cooled icebox to the Laboratory of Microbiology, Educational Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Bacteriological examination and serotyping of E. coli isolates
Using Irwin et al. (2010) method, the collected tissue samples were homogenized in sterile saline solution at 10%. Then 0.1 mL of the homogenate was platted on MacConkey agar plates (Difco, Detroit, MI, USA), followed by incubation at 37 °C for 18-24 h. Lactose-fermenting colonies (Pink, round medium-sized colonies) were re-inoculated onto eosin methylene blue agar plates (Difco, Detroit, MI, USA). Typical colonies of E. coli appeared greenish, metallic with dark purple center. Colonies were kept on Nutrient agar slants at 4 °C for further identification. Based on staining and biochemical tests, identification of E. coli isolates was done (Cloud et al., 1985).
The confirmed E. coli isolates were serologically identified using rapid diagnostic E. coli antisera sets (Difco, Detroit, MI, USA) for diagnosis of the APEC serotypes (Kok et al., 1996).
DNA extraction and detection of virulence associsted genes by multiplex polymerase chain reaction (PCR)
Bacterial DNA was extracted from each of glycerol stock serotyped E. coli according to the previously described method (Ghanbarpour et al., 2010). The concentration and the purity of the extracted DNA was evaluated by Nanodrop.
A multiplex PCR was conducted to detect the virulence-associated genes of E. coli. The tested virulence-associated genes were included temperature-sensitive hemagglutinin (tsh), Arginine succinyltransferase (astA) and iron outer membrane receptor (iroN). Primer sets (Table 1) Eldin (2019). PCR assays were performed on a Thermal Cycler (Master cycler, Eppendorf, Hamburg, Germany) using the method of Eldin (2019). Amplified DNA fragments were analyzed by 2% of agarose gel electrophoresis (Applichem, Germany, GmbH). The reference strains were E. coli O157:H7 Sakai (positive control strain) and E. coli K12DH5α (a nonpathogenic negative control strain) kindly provided by Professor Mohamed Hassan, Food Control Department, Benha University, Egypt.
Antibiogram of the identified E. coli serotypes
Identified E. coli serotypes were subjected to antibiogram susceptibility test using the disk diffusion technique and antimicrobial resistance profile were examined according to Clinical and Laboratory Standards Institute (CLSI) guidelines (2013). The inhibition zone diameter for each antimicrobials was measured and interpreted according to CLSI guidelines (2013).
RESULTS AND DISCUSSION
Prevalence of E. coli in ducks
The affected ducklings under investigation have a history of poor growth and diarrhea with respiratory signs in some cases with clinical postmortem findings of air sacculitis, pericarditis, perihepatitis, peritonitis and erosions on the gizzards. The overall isolation rate of E. coli in the examined ducks was 34.5% which considered high compared to the isolation rates of E. coli from diseased ducks in Egypt (22.8%) in pervious study Eid et al. (2019), while lower than Na et al. (2019) who reported 36.13% in ducks in
Table 1: Sequence and specificity of PCR primers and their product sizes
| Target | Primer sequence (5’-3’) | Product size (bp) | Tm (°C) | Accession number | Reference |
|
Temperature-sensitive hemagglutinin (tsh) |
F-5’-AATAATGCGCCGTCACTGG-3’ R-5’-AAGGAGCGCTATCCTGTTT-3’ |
284 | 60 | JX466850.1 | Eldin (2019) |
|
Arginine succinyltransferase (astA) |
F-5’-CCAAAAACCTCAAAACCCC-3’ R-5’-TATGCCAAAGGGATGACCA-3’ |
422 | 60 | NC_000913 | |
|
iron outer membrane receptor (iroN) |
F-5’-TCGGTATGGTTTGATTCC-3’ R-5’-CAATGGCCGTACGTCCTA-3’ |
617 | 60 | NC_014615 |
South Korea. In China E. coli isolation rate in ducks was (47.1%) Liu et al. (2018) while higher prevalence rate (69.1%) was reported in chicken Wu et al. (2014). The higher isolation rate was recorded in ducklings aged 1-2 weeks 66.66% (12/18), followed by 3-4 weeks 50% (5/10), 5-6 weeks 42.86% (3/7) and ducks of 7-8 weeks 40% (2/5), respectively (Figure 1). This rate indicates that the incidence of the disease is high in the young age compared with the older ducks. Similar results were reported in ducks from Italy (Sgariglia et al., 2019).
The prevalence rate of APEC among different examined tissues revealed that liver had the highest detectable rate 50% (20/40), followed by spleen 42.5% (17/40), heart 30% (12/40), lungs 25% (10/40) and gizzards 25% (10/40), respectively (Figure 2). The dissemination of E. coli in different organs such as liver, lungs and heart is verified by postmortem lesions including fibrinous perihepatitis, pericarditis, enteritis and pneumonia indicating that E. coli led to septicemia and followed by death of the birds (Kabir, 2010). The tissue distribution of APEC is in correspondence with that reported in ducks (Darwish et al., 2015) and quails in Egypt (Eldin, 2019) reported that liver harbored the highest rate of the extra-intestinal APEC. Additionally, E. coli was isolated from different tissues (spleen, liver, kidney, trachea, lungs, skin, ovary, oviduct, intestine, and cloaca) of chicken and turkeys in Brazil (De Carli et al., 2015). The high prevalence rate of

Figure 2: Tissue distribution of the identified E. coli serotypes isolated from ducks. Incidence (%) of the identified E. coli serotypes from different tissues of the diseased ducks collected from Sharkia Governorate, Egypt (n=40).
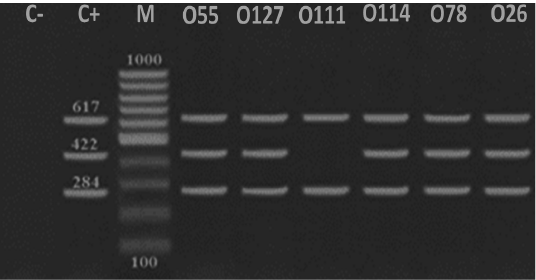
Figure 3: Detection of DNA virulence-associated genes in E. coli serotypes isolated from ducks. A representative DNA gel electrophoresis image for a multiplex PCR reaction for virulence-associated genes including tsh (284 bp), astA (422 bp) and iroN (617 bp) in E. coli serotypes (O55, O127, 0111, O114, O78 and O26). M refers to a 100 bp DNA marker, C- refers to a negative control while C+ refers to a positive control.
E. coli in the liver might be attributed to the translocation
Table 2: Antimicrobial resistance profile among the identified E. coli serotypes
|
E. coli O55:H7 |
E. coli 127:H6 |
E. coli O111:H4 |
E. coli O114:H21 |
E. coli O78:H- |
E. coli O26:H11 |
|||||||
| Ampicillin | 17 | 89.47 | 12 | 85.68 | 6 | 85.68 | 8 | 72.72 | 5 | 100 | 13 | 100 |
| Ceftiofur | 6 | 31.58 | 2 | 14.28 | 2 | 28.56 | 1 | 9.09 | 2 | 40 | 6 | 46.15 |
| Chloramphenicol | 3 | 15.79 | 2 | 14.28 | 3 | 42.84 | 2 | 18.18 | 1 | 20 | 2 | 15.38 |
| Ciprofloxacin | 5 | 26.32 | 1 | 7.14 | 2 | 28.56 | 1 | 9.09 | 2 | 40 | 5 |
38.46 |
| Enrofloxacin | 4 | 21.05 | 1 | 7.14 | 3 | 42.84 | 2 | 18.18 | 2 | 40 | 3 | 23.07 |
| Erythromycin | 4 | 21.05 | 3 | 21.42 | 1 | 14.28 | 2 | 18.18 | 2 | 40 | 3 | 23.07 |
| Gentamicin | 2 | 10.53 | 2 | 14.28 | 1 | 14.28 | 1 | 9.09 | 1 | 20 | 2 | 15.38 |
| Lincomycin | 7 | 36.84 | 2 | 14.28 | 3 | 42.84 | 1 | 9.09 | 0 | 0 | 2 | 15.38 |
| Nalidixic acid | 14 | 73.68 | 12 | 85.68 | 5 | 71.4 | 8 | 72.72 | 3 | 60 | 9 | 69.23 |
| Neomycin | 4 | 21.05 | 2 | 14.28 | 1 | 14.28 | 1 | 9.09 | 1 | 20 | 2 | 15.38 |
| Oxytetracycline | 8 | 42.10 | 4 | 28.56 | 3 | 42.84 | 3 | 27.27 | 2 | 40 | 8 | 61.54 |
| Penicillin | 19 | 100 | 12 | 85.68 | 7 | 99.96 | 10 | 90.9 | 5 | 100 | 13 | 100 |
| Polymyxin B | 15 | 78.94 | 10 | 71.4 | 4 | 57.12 | 8 | 72.72 | 5 | 100 | 10 | 76.92 |
| Sulpha-trimethoprim | 8 | 42.10 | 5 | 35.7 | 2 | 28.56 | 4 | 36.36 | 3 | 60 | 7 | 53.84 |
| Number of isolates | 19 | 100 | 14 | 100 | 7 | 100 | 11 | 100 | 5 | 100 | 13 |
100 |
of E. coli pathotypes from intestine to the liver through the mesenteric lymph nodes leading directly for failure of the immune defense (MacFie, 2004).
Sixty-nine E. coli strains were isolated in the current study. Serotyping of the isolated E. coli revealed six serotypes, namely E. coli O55:H7, E. coli O127:H6, E. coli O111:H4, E. coli O114:H21, E. coli O78:H- and E. coli O26:H11. E. coli O55:H7 had the highest isolation rate 27.5% (19/69 strains), followed by E. coli 127:H6 20.3% (14/69), E. coli O26:H11 18.8% (13/69), E. coli O114:H21 15.9% (11/69), E. coli O111:H4 10.1% (7/69) and E. coli O78:H- 7.24% (5/69), respectively (Figure 2). Similarly, Darwish et al. (2015) isolated and identified E. coli O26, O78, O114 and O127 serotypes from ducks in Egypt. On the other hand, Cloud et al. (1985) noted that E. coli O2, O35 and O78 are frequently associated with avian colibacillosis.
Detection of virulence-associated genes
Avian pathogenic E. coli strains that cause colibacillosis harbor a peculiar set of pathogenicity genes that called virulence factors which facilitate the dissemination of the bacteria into different organs and colonization (Alizade et al., 2017; Dho-Moulin and Fairbrother, 1999). Temperature-sensitive hemagglutinin (tsh) is a member of the autotransporter group of protein strains harbored this virulent factor are characterized by their high pathogenicity and lethality as it contributes to the development of air sacculitis, pericarditis, perihepatitis and peritonitis (Dozois et al., 2000). Our results indicated that all identified pathotypes harbored tsh and iroN clarifying the importance of these genes for pathogenicity while E. coli O55, O127, O111, O114, O78 and O26 serotypes convoyed tsh and iroN (Figure 3). On the other hand, Darwish et al. (2015) and Dozois et al. (2000) detected these virulent factors at E. coli O1, O2, O26, O78, O86, O114 and O127 serotypes that isolated from chickens and ducks. Iron outer membrane receptor (iroN) is another virulent factor that facilitate iron uptake, pathogenicity and biofilm formation (Magistro et al., 2015). Also Darwish et al. (2015) and Eldin (2019) highlighted that E. coli strains isolated from different organs of ducks and quails including O26, O78, O86, O114 and O127 extracted iroN. Likewisem arginine succinyltransferase (astA) is associated with faster growth and rapid multiplication of different species of bacteria including E. coli (Schneider et al., 1998). In the present study, astA was expressed in E. coli O55, O127, O114, O26 and O78 serotypes (Figure 3). Similarly, astA was expressed in E. coli O157 serotype (Shirai and Mizuguchi, 2003), E. coli O78, O86 and O114 serotypes (Darwish et al., 2015).
Antibiogram of the identified E. coli serotypes
The excessive use of antimicrobials in poultry farms in Egypt had led to development of antimicrobial-resistant bacterial strains that resulted in a clear difficulty in the control of such pathogens (Darwish et al., 2013).
The resistance profiles for the identified E. coli pathotypes in the present work was reflected that E. coli O55 was resistant 100% to penicillin; 89.47% to ampicillin 78.94% to polymyxin B; 73.68% nalidixic acid and less than 50% to other tested antimicrobials (Table 2). E. coli O127 had a resistance profile as follows: 85.68% to ampicillin, penicillin, nalidixic acid; 71.4% to polymyxin B, while this serotype showed resistance percentage to the other tested antimicrobials from 7.14% to 35.7% (Table 2). E. coli O111 was resistant 100% to penicillin, 85.68% to ampicillin and 57.12% to polymyxin B. While were less resistant to erythromycin, gentamicin and neomycin (14.28%). The resistance profiles of E. coli O114 were as following: 90.9% to penicillin; 72.72% to each of ampicillin, nalidixic acid and polymyxin B; 36.36% to sulpha-trimethoprim and 9.09% to each of ceftiofur, ciprofloxacin, gentamycin, lincomycin and neomycin (Table 2). E. coli O26 and O78 showed complete resistance to ampicillin, penicillin and polymyxin B (Table 2). It is clear from our results that most identified E. coli serotypes showed marked sensitivity to some of the tested antibiotics including gentamycin, neomycin, enrofloxacin and erythromycin. Our results are comparable with that reported by Darwish et al. (2015) who reported that E. coli O26 and O78 were highly sensitive to cefotaxime and norfloxacin but resistant to amoxicillin. Na et al. (2019) isolated extended-spectrum β-lactamase producing E. coli isolated from ducks in South Korea. Furthermore, Farghaly et al. (2017) declared that E. coli O20, O78 and O127 isolated from quails showed marked resistance to amoxicillin (71.4%), ciprofloxacin (57.1%), and nalidixic acid (57.1%). The excessive and massive use of the antibiotics in the poultry production might explain the emergence of such resistance to E. coli strains. Therefore, the misuse of antibiotics should be stopped in duck farms in Egypt. Future approaches are also needed to investigate the expression of genes related to antimicrobial resistance in the identified E. coli serotypes.
Conclusion
The current study indicates the prevalence of E. coli infection in ducklings and ducks in Egypt. Ducklings are highly susceptible to E. coli infection compared with old ducks and liver is the most affected organ. The prevalent E. coli serotypes were E. coli O55:H7, E. coli O127:H6, E. coli O111:H4, E. coli O114:H21, E. coli O78:H- and E. coli O26:H11, respectively, and harbored virulence-associated genes tsh and iroN. All extracted astA gene except E. coli O111:H4. The identified E. coli had multidrug resistance profiles while gentamycin, neomycin and lincomycin are considered as promising candidates for the control of E. coli infection in ducks in Egypt.
Authors Contribution
All authors contributed equally.
Conflict of interest
There is no conflict of interest.
REFERENCES