Advances in Animal and Veterinary Sciences
Research Article
Evaluation of the Protective Effects of Adsorbent Materials and Ethanolic Herbal Extracts against Aflatoxins Hepatotoxicity in Albino Rats: Histological, Morphometric and Immunohistochemical Study
EL-Shaymaa EL-Nahass 1*, Walaa A Moselhy2, Nour El-Houda Y. Hassan2, Atef A. Hassan3
1Department of Pathology, 2Department of Toxicology and Forensic Medicine, Faculty of Veterinary Medicine, Beni-Suef University, 62511, Egypt. 3Department of mycology and mycotoxins, animal health research institute, Cairo, Egypt.
Abstract | Aflatoxins have potent toxic effects on various organs mainly liver. This study aimed to evaluate the protective effect of certain adsorbent materials (halloysite and chitosan) and ethanolic herbal extracts, Moringa olifera and Thymus vulgaris extracts, on the aflatoxin-induced hepatotoxicity. 30 adult rats were divided into 6 groups: control negative group (GI), rats received aflatoxins (GII), rats received aflatoxins and halloysite(GIII), rats given aflatoxins and chitosan (GIV),rats received aflatoxins and ethanolic extract of Moringa olifera (GV) and rats received aflatoxins and ethanolic extract of Thymus vulgaris (GVI). Specific histo-morphometric analyses were done for HE stained hepatic tissues, Masson’s trichrome and immunohistochemistry. The main pathological lesions were degenerative changes and necrosis especially in GII. Morphometric analysis revealed the presence of a clear focal cytoplasmic acidophilia (red color) in GII and being significantly decreased in GIII, GIV, GV and GVI. A significant decrease in nuclear chromatin area percentages (blue color) could be found in GII (3.4%), GV (4.0%) and GVI (5.1%) compared to the control negative group (6.5%). Meanwhile, GIII and GIV revealed a significant improvement in nuclear chromatin area percentage compared to GII. Quantitative analysis of integrated intensities of positive immunohistochemical reactions of caspase-3 and TNF-α showed the highest intensities aflatoxin-fed group (GII). In other treated groups, a significant decrease in caspase-3 and TNF-α expression was detected. In conclusion, the present study showed the protective effect on the use of adsorbent materials and the ethanolic extracts of Moringa Olifera and Thymus vulgrais for mitigating the deleterious effects of hepatotoxicity induced by aflatoxins.
Keywords | Aflatoxins, Adsorbents, Herbal extract, caspase-3, TNF-α.
Received | June 25, 2019; Accepted | August 19, 2019; Published | November 26, 2019
*Correspondence | El-Shaymaa El-Nahass, Associate Professor of Pathology, Faculty of Veterinary Medicine, Beni-Suef University, 62511, Egypt; Email: shima_k81@yahoo.com
Citation | El-Nahass S, Moselhy WA, Hassan NHY, Hassan AA (2019).Evaluation of the protective effects of adsorbent materials and ethanolic herbal extracts against aflatoxins hepatotoxicity in albino rats: histological, morphometric and immunohistochemical study. Adv. Anim. Vet. Sci. 7(12): 1140-1147.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.12.1140.1147
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 El-Nahass et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
It is globally reported that a considerable amounts of food crops are contaminated with mycotoxins; these toxins are secondary metabolites produced by several filamentous fungi including the genera Aspergillus, Fusarium, and Penicillium. Mycotoxins are chemically and thermally unchangeable, that renders them to be resistant and stable in the processing of various feed manufacturing techniques (Pietsch, et al., 2013). Aflatoxins B1 (AFB1) are the major class of mycotoxins produced by Aspergillus flavus and Aspergillus parasiticus (Hussain et al., 2010).
Mycotoxins cause serious public health risk effects since they have carcinogenic, teratogenic, nephrotoxic, and hepatotoxic effects following their consumption in the contaminated grains/animal food products (Greco et al., 2014) resulting in significant economic losses in the food industry due to decreased reproductive performances and increased mortalities. (Galarza-Seeber et al., 2016).
Using of adsorbents in previous trials has been made to detoxify the contaminated feedstuffs/diets with mycotoxins in order to reduce the condition (Döll and Dänicke, 2004). Among them, the physical treatment by the use of thermal and irradiation inactivation, chemical treatment by ozonation and ammoniation and biological methods through bacterial degradation or adsorption were common (Jouany, 2007). The later is considered to be one of the most effective methods by the use of adsorbents in feed stuffs including activated carbon (Binder 2007) and kaolin (Figueroa, et al., 2004), Chitosan (Solís-Cruz et al., 2017) and Halloysite (Zhang, et al., 2014).
Several herbal extracts from leaves, seeds, roots and berries including Moringa olifera, Thymus vulgaris, ginger, turmeric, saffron, coriander, cloves are an excellent source of natural antioxidants like essential sulfur-containing amino acids, flavonoids, minerals and other compounds with promising health beneficial effects (Kabakand Dobson, 2017). Moringa oleifera leaf extract antioxidants have strong properties as antimutagenic, anticarcinogenic, anti-inflammatory, and antifungal agent (Sathya et al., 2010). Moreover, antioxidants are commonly used to detoxify the effect of mycotoxins in the contaminated food. Natural antioxidants were commonly to reduce the adverse lesions induced by these toxicants (Tingting et al., 2015).
Several techniques have been used to determine the adverse effects of infectious agents and toxins. Histopathological examinations, including IHC and computer-assisted image analysis, are encouraged as new tools for high-precision measuring the size and shape of cell nuclei in biological laboratories (Deans et al., 1993). A lot of these profiles seemed to be valuable and sensitive in the predictive judges particularly in human malignancies lesions (Jalava et al., 2001). Using of immunohistochemistry is a valuable technique for confirmation of many pathological lesions. Caspases chew up the cellular constituents in apoptosis into non-inflammatory molecules following toxic conditions (Martin et al., 2012). The later induces activation of caspase 3 in hepatocytes (Michitaka et al., 2012), stimulating the formation of apoptotic cells which characterized by intense caspase 3 reaction which is mainly associated with Tumor Necrosis Factor (TNF) receptors stimulation (Min el., 2014).
The aim of the current study was to evaluate the ameliorative effects of adsorbents materials including halloysites and chitosan, and certain alcoholic extracts, Moringa olifera and Thymus vulgaris in reducing the heptotoxicity of aflatoxins in albino rats.
Materials and Methods
Materials:
Aflatoxigenic strains: Aflatoxins were obtained from the fungus, Aspergillus flavus, at laboratory of Mycology and Mycotoxins, Animal Health Research Institute, Agriculture Research Centre (Smith, 1997).
Preparation and detection of aflatoxin by thin layer chromatography: The aflatoxigenic isolates of A. flavusre revealed from feed were inoculated into containers with 50 ml of sterile yeast extract solution 2% and 20% sucrose. Incubation occurred at 25 ºC for 15 days. Contents were filtered and mycelial mat were separated from YES medium at the end of the incubation. Both were measured for the detection of aflatoxins qualitatively by the use of thin layer chromatography (TLC). Aflatoxins- positive samples were assessed quantitatively by fluorometric method using specific FGisAfla test standards (AOAC, 2000; Refai and Hassan, 2013). The aflatoxigenic A. flavus strains obtained from food samples were screened for the presence of aflatoxins on yellow corn (Smith, 1997). Accordingly, 100gmwell-grounded yellow corn and 40-50 ml distilled water were mixed, autoclaved at 121C for one hour and shacked to prevent cooking of yellow corn. Inoculation with spore suspension of 2 slants of A. flavus for 4 weeks at 25-28C was done. Then, the corn was removed, dried and well-grounded.Fifty grams of each were subjected to TLC for qualitative estimation of aflatoxins (AOAC, 2000; Refai and Hassan, 2013).
Halloysite and chitosan preparation: Halloysites nanoclay (Formula: H4Al2O9Si2· 2H2O) and Chitosan (molecular weight: 100,000-300,000.) were purchased from Sigma-Aldrich Chemical Co., USA.
Ethanolic extraction of Moringa olifera and Thymus vulgaris: Moringa oleifera and Thymus vulgaris leaves were obtained from the Egyptian Scientific Society of Moringa farm. Taxonomic identification was done by the National Research Center; Giza, Egypt. The powdered plant was soaked in adequate volume of ethyl alcohol 70%. Extraction was done by intermittent shaking at room temperature for 3 days. Filtration through a filter paper (Whatman No. 4) and the residue was re-extracted twice, and it was evaporated under reduced pressure using a rotatory evaporator. The extract was dried to obtain a constant weight and it was stored at 4°C until use (Williamson et al., 1996)
Experimental Animals
Thirty mature male albino rats, weights 150±20 g, were supplied from the breeding unit of laboratory animal, Faculty of Veterinary Medicine, Beni-Suef University. All animals were housed in polypropylene cages, placed in a ventilated animal house, suitable temperature, relative humidity, and 12-h light/dark cycle, and properly maintained. Prior to experiment, rats fed on healthy commercial food pellets for rats and water was available ad libitum. The rats were acclimated to the environment for ten days. The rules of the ethics committee of Faculty of Veterinary Medicine, Beni-Suef University were followed (Institutional Animal Care and Use Committee, Beni-Suef University).
Experimental Design
Rats were randomly assigned into six groups (5 of each). Group I receive distilled water during the whole period of experiment (30days) and served as (−ve) control. Group II received 0.5 ppm of aflatoxin in feed (+ve) (Atef et al., 2010). Group III received aflatoxins and hallosite 350 mg/kg BW using oral gavage (Xue Wang et al., 2018) after dose conversion by Paget and Bernes table (Paget and Bernes 1964). Group IV received aflatoxins and oral intubation with chitosan 200mg/kg BW (Ozcelik et al., 2014). Group V received aflatoxins and 400 mg/kg b.w of Moringa olifera extract using oral gavage (Singh, et al., 2014). Group VI received aflatoxins and 300 mg/kg b.w of Thymus vulgaris extract using oral gavage (Shimaa and Emad, 2014).
The symptoms and mortality of rats were observed every day and the weights of rats were recorded. After last oral administration, rats were fasted, anaesthetized with ether alcohol and chloroform mixture. Tissue specimens of rats were collected.
Histopathology
Liver specimens were prepared and fixed in 10% neutral buffered formalin for 48 hours followed by routine processing and microtomy of 4-6 μm sections. Staining with hematoxylin and eosin (HE) and Masson’s trichrome to identify the fibrous connective tissues; mainly collagen fibers (Bancroft et al., 1991).
Immunohistochemical Expression of Caspase-3 and TNF-α Expression in Hepatic Tissues
Immunohistochemical labelling of casapse-3 and TNF-α on 4 μm thick paraffin-embedded sections was carried out on liver tissues of all experimental rats. Sections were dewaxed and rehydrated in graded ethanol. Antigen retrieval was performed to expose target proteins, using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. \ Following the antigen retrieval, endogenous peroxidases were blocked using 3% hydrogen peroxide-methanol for 30 min at room temperature. Incubation of primary antibodies including caspase-3 Mouse monoclonal, Santa Cruz biotechnology, Sc-7272) and TNF-α (Mouse monoclonal, Santa Cruz biotechnology, Sc-130347) in a dilution of 1:500 overnight at 4°C in a humidified chamber was done. Washing of the sections and incubated with HRP-conjugated secondary antibody (EnVision + System HRP-labelled polymer anti-rabbit; Dako®) for 60 minutes at RT. The final step is the addition of 3,3-diaminobenzidinetetrahydrochloride (Liquid DAB + Substrate Chromogen System, Dako®). Tissues were counterstained with Mayer’s hematoxylin, dehydrated, cleared and mounted for examination.
Micropathomorphologic Analyses
Hepatic histo-morphometric analyses were performed by using optical microscope. Images were captured by a digital camera (Leica, DM2500 M). A freeware version of Image-J (1.45s) was downloaded from the NIH website and used for image analysis.
Measurements of area percentages of color intensities of routinely HE stained slides: Quantification was carried out by using twenty microscopic fields (x200 magnification) for each group that corrected by enhance contrast (at level of 0.4) for the calculation of both red and blue color area percentages. The CMYK stack splitter in image J software was used in order to convert RGB colored images to 4-slices (cyan, magenta, yellow and K for black) stacks. The yellow stacks were used to set the boundaries of the cytoplasm of hepatocytes while the cyan stacks were used to set the boundaries of the nuclear chromatin.
Measurements of collagen fibers area percentages: Quantification was performed using Masson’s trichrome stain. Twenty microscopic fields (x200 magnifications) were randomly selected and evaluated.
Measurements of positive immunohistochemical reactions TNF-α and caspase-3: The positive immunohistochemical cytoplasmic reactions for TNF-α and caspase-3 were quantified in ten microscopic fields (x200, x400 magnifications) by using HSB (hue, saturation, and brightness).Stack splitter was used to converts RGB colored images to 3-slice (hue, saturation, and brightness) stacks. The brightness stacks were used to set the boundaries of the positive brown immunohistochemical reaction then thresholded by red binary color to include all reactions.
Statistical Analysis
The statistical evaluation was done by SPSS version 21 software package (SPSS, Inc, USA) through one way ANOVA followed by Dunnett tests for control negative group (G1) comparison. P value ≤ 0.05 was considered statistically significant. All data were tabulated as Means± SD.
Results
No gross lesions could be found on the hepatic structure of rats in all groups, in addition no mortalities could be found.
Quantification of Histopathological Lesions using H&E stain
Area percentages of red color intensities of the hepatic cytoplasm: Normal histological structure of the liver could be found in GI group, the area% of red color intensity was 36.5% (Fig. 1a-3a). GII showed severe coagulative necrosis which indicated by increasing of the cytoplasmic acidophilia to reach 45% (Figs 1b-3b). GIII, GIV, GV and GVI showed significant decreasing in the cytoplasmic red color intensities (35-37%) in comparison with aflatoxins feed group (GII). (Table 1, Figs. 1c-3c, 1c-1d-3d, 1e-3e, 1e-4e)
Area percentages of blue color intensities of hepatic nuclear chromatin: Detailed values of the percentage of hepatic nuclei were summarized in Table 1. The area percentages of nuclear chromatin in the examined fields were 6.5, 3.4, 7.2, 7.5, 4 and 5.2 in GI, GII, GIII, GIV, GV and GVI, respectively. A significant reduction in was found in GII, GV and G6. On the other hand, no significant difference could be found in G3 and G4 compared to the control negative group (Figs. 1a-1f, 3a-3f)
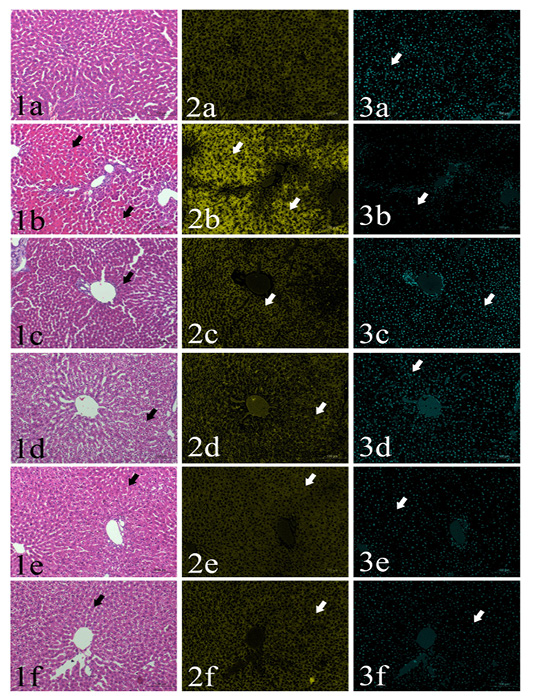
Plate (I), Figs. (1-3): SSections of rat’s liver routinely stained with Hematoxylin and Eosin stain (1st column) showed focal cytoplasmic acidophilia (arrows) in different groups including GI (1a), GII (1b), GIII (1c), GIV (1d), GV(1e) and GVI (1f). HE x200, and their corresponding yellow stacks (2nd column) used for measuring cytoplamic color (arrows) in different groups including GI (2a), GII (2b), GIII (2c), GIV (2d), GV (2e) and GVI (2f), and Cyan stacks (3rd columns) used for measuring nuclear chromatin area percentages (arrows) in different groups including GI (3a), GII (3b), GIII (3c), GIV (3d), GV (3e) and GVI (3f),
Quantification of Fibrous Connective Tissue Area Percentages using Masson’s Trichrom Stain
The area percentage of collagen fibers ranged from 2.8% to 3.3% in all groups with no significant difference among groups (Table 1, Figs.4a-4f).
Quantification of Integrated Intensities and Area Percentages of Positive Immunohistochemical Reactions of Caspase-3 and Tnf-Α
Cytoplasmic immunoreactivity of TNF-α was observed in the hepatocytes and activated Van Kupffer cells (Table 1, Figs.5a-5f). The highest area percentages as well as integrated intensities were detected in GII in comparison to other groups. Meanwhile, no significant differences could be found among GI, GIII, GIV, GV and GVI.
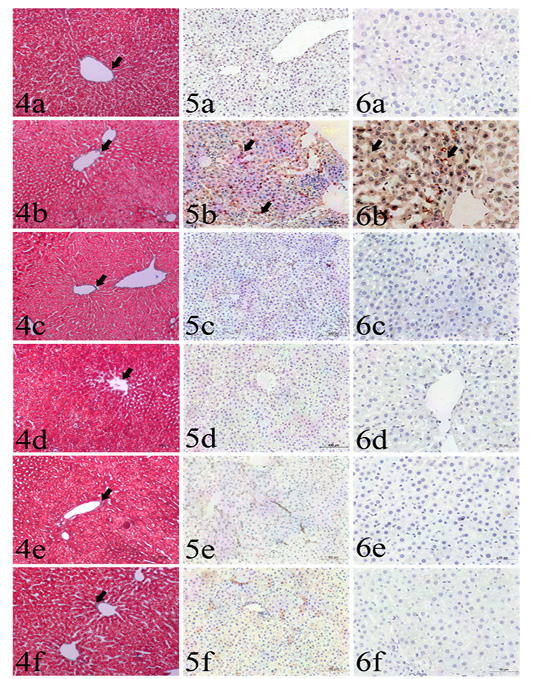
Plate (II), Figs. (4-6): Sections liver stained with: Fig. (4): Masson’s trichrome from different groups used for collagen fibers identification (arrows), including GI (4a), GII (4b), GIII (4c), GIV(4d), GV (4e) and GVI (4f). Masson’s trichrome x200. Fig (5): Caspase 3 immunohistochemistry expression (arrows) among the examined groups including GI (5a), GII (5b), GIII (5c), GIV (5d), GV (5e) and GVI (5f). IHC x200. Fig. (6): TNF-α immunohistochemistry expression (arrows) among the examined groups including GI (6a), GII (6b), GIII (6c), GIV (6d), GV (6e) and GVI (6f). IHC x400.
Table 1: Quantitative histo-morphometrical analysis using H&E, Masson’s trichrome stained sections and immunohistochemistry.
| GVI | GV | GIV | GIII | GII | GI | Measured parameter |
| H&E stained section | ||||||
| 37.3±11.6 | 36.5±12.8 | 35.6±6.1 | 37.0±6.2 | 45±7.5* | 36.5±12.3 | Area percentages (%) of red color intensities |
| 5.2±1.2* | 4±0.8* | 7.5±2.2 | 7.2±1.5 | 3.4±1.3* | 6.5±0.6 | Area percentages (%) of blue color intensities |
| Masson's trichrome stained sections | ||||||
| 3.3±1.1 | 3.2±1.8 | 3.3±1.2 | 2.8±1.9 | 3.2±1.4 | 3.2±1.6 | Fibrous connective tissues area% |
| Immunohistochemistry (IHC) | ||||||
|
945846±5661
|
958290±3233
|
1195185±6502
|
947388±4296
|
12066039±2320*
|
677407±4433
|
Caspse-3 positive reactions integrated intensities (Pixels) |
| 4004877±2791 | 5761878±3742 | 1594260±1678 | 3813729±3007 | 11244582±7045* | 636429 ±1944 |
TNF-α positive integrated intensity (Pixels) |
* Significant difference in comparison to control negative group (GI), P<0.05
The highest integrated intensities of positive cytoplasmic immunohistochemical reactions of caspase-3 were clearly detected in GII (Table 1, Figs. 6a-6f). The other treated groups (GIII, GIV, GV and GVI) had occasional weak cytoplasmic expression, with no significant differences compared to the control negative group (GI).
Discussion
Lots of biological toxins are found in the natural environment. These toxins are basically dangerous for both human and animal health. Mycotoxins considered being the most hazardous ones. They are secondary toxic metabolites produced by certain filamentous fungi, belonging to genera Aspergillus, Penicillium and Fusarium (Charmley, et al., 1994, Ostryl et al., 2017). Chemically, these toxins are a heterogeneous group of compounds, including over than 20 groups. The most toxic and common types are aflatoxins B1 (ABB1). Aflatoxins G1, B2 and G2 are lesser toxic (Freire and Sant’Ana, 2018).
Aflatoxins are dangerous and have severe adverse effects on various tissues. The most important one is the ability to produce the carcinogenic effects following the consumption of contaminants of food (Negash, 2018). The exposure of high dose of aflatoxins may cause vomiting, abdominal pain, and even death. Furthermore, the chronic exposures to small quantities mainly cause liver cancer (Sherif et al., 2009). A previous study was carried out to investigate pathological lesions in the internal organs, particularly the liver. In the later, the predominant hepatic lesions consisted of focal areas of coagulative necrosis, vacuolar and hydropic degeneration associated with mononuclear cellular aggregations (Bakeer et al., 2013). However, this study didn’t perform morphometric analysis for quantification of lesions. Application of morphometric analysis was done to quantify the percentages area of cytoplasmic acidophilia. The highest percentages were found in GII and being decreased in GIII, GIV, GV, and GVI. Interestingly, a significant reduction in nuclear chromatin area percentages was found in GII compared to the control negative group. GIII and GIV revealed a significant improvement in these percentages compared to GII.
The most important and practical dietary approach for prevention of mycotoxicosis in animals might be attributed to the use of adsorbents (Watts et al., 2003; Wang, et al., 2006, Kana et al., 2013). Several inorganic materials and clay, including bentonites have the adsorptive properties to reduce the toxic and adverse effect of aflatoxins. Meanwhile, some of these materials have a strong potential effect to adsorb mycotoxins in feed (Rawal et al., 2010, Khan et al., 2014).
A previous study revealed that four adsorbent materials are tested against mycotoxins, including biopolymer chitosan (CHI) and three cellulosic polymers, in poultry. Such adsorbents have the advantage of being them act in a wide range of pH in the digestive tract (Solís-Cruz et al., 2017)
Another potent adsorbent is the hydrated sodium calcium aluminosilicate (HSCAS) which is firstly used, both in vitro and in vivo, as a sequestrant in the gastrointestinal tract (Phillips et al., 1987). Similar study reported that the use of various concentrations of HSCAS (0.1% and 0.5%) in chicken diets which fed an aflatoxin-contaminated feedstuff was able to reduce the bioavailability of aflatoxins (Davidson et al., 1987). Concomitantly, a previous literatureon the goat dietrevealed that the addition of HSCAS to the lactating dairy goats which are previously exposed to various concentrations of aflatoxins (100/200 ppb) led to a significant reduction of aflatoxins residues in milk (Smith et al., 1994). In the agreement with the previous results, our study showed that the adsorbents-treated groups (GIII and GIV) showed a significant improvement in the nuclear chromatin and normal expressions of TNF-α and caspase-3 (in comparison to control negative group GI). In parallel, Avantaggiato, et al., detected that carbon/aluminosilicate-based products have fast binding and adsorption properties for zearalenone within the gastrointestinal tract leading to reduction of the adverse effects of zearalenone on various internal organs (Avantaggiato et al., 2007).
Previous toxicological studies explained the beneficial effects of certain herbal extracts, like the green tea, against the adverse effects of variable damaging agents on internal organs (liver, kidneys and brain) (Khalaf et al., 2012). Recently, Moringa olifera extract has a great role in the treatment of several diseases acting as antibiotic, anti-inflammatory and hypoglycemic (Hamza, 2010). Currently, the use of adsorbents (hallosites and chitosan) and ethanolic extracts of Moringa olifera and Thymus vulgaris had a significant decrease in TNF-α and caspase-3 expressions, compared to aflatoxins feed group (GII). In the later group (GII) these activation might attributed to activation of apoptosis within the liver cells by the hypoxia toxic conditions which mainly associated with TNF-receptor activation that induces apoptosis resulting in extensive increase in caspase 3 activity. (Michitaka et al., 2012; Min et al., 2014).
The ameliorative effects of Moringa olifera extract might be attributed to its anti-oxidative activity and for its ability for scavenging/suppression the generation of free radicals and toxins leading to the minimization the of lipidperoxidation intensity with enhancement of the antioxidant enzymes activities (Maduka et al., 2014).
Conclusion
In the current work, the hepatic damage caused by aflatoxins was recognized by degenerative changes, necrosis of hepatic tissues associated with a significant decrease in the nuclear area indicating the nuclear damage and a significant increase in cytoplasmic acidophilia. A significant decline in the cytoplasmic acidiophilia could be detected in adsorbent-treated groups (GIII and GIV) and ethanolic extracts of Moringa olifera and Thymus vulgaris treated groups (GV and VI) as well as a significant improvement in the nuclear area percentages could be detected in adsorbent-treated groups (GIII and GIV).
Acute aflatoxicosis induced a significant increase in TNF-α and caspase-3 expressions in livers, these expressions were significantly decreased in all treated groups either by adsorbents or herbal extracts confirming their protective effects against the adverse effects of mycotoxins. Thus, the use of adsorbents, halosites and chitosan, protect the liver against experimental aflatoxicosis. Meanwhile, the use of herbal alcoholic extracts of Moringa olifera and Thymus vulgaris produced lesser protection against hepatotoxicity by decreasing immunohistochemical expression of TNF-α and caspase-3 expression.
Although halloysite had an effective reduction of hepatotoxicity in albino rats, no studies have been performed to study details of the possible long-term effects of these materials on the utilization of essential nutrients like vitamins and minerals. Further investigations are recommended to evaluate the long term administration as well as the dosage of these adsorbents.
Acknowledgement
Authors would greatly like to express gratitude to Project Support and Development Unit, Beni-Suef University
Conflict of interest
Authors declare that there is conflict of interest.
authors contribution
All authors contributed equally.
References
selenite against aflatoxin B1 on hemoglobin content, erythrocyte count, immune adherence function in broilers. Indian J. Anim. Res. 49:360–363 https://doi.org/10.5958/0976-0555.2015.00047.3





