Advances in Animal and Veterinary Sciences
Research Article
Efficacy of a Recombinant Vaccine Against Pasteurellosis in Chickens and Ducks
Didik Handijatno1, Nur-Aliyah Ahmad2, Sabri Md Yusoff3, Annas Salleh2, Mohd Zamri-Saad2
1Department of Microbiology, Faculty of Veterinary Medicine, Airlangga University,60115 Surabaya, Indonesia; 2Department of Veterinary Laboratory Diagnosis, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Malaysia; 3Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Malaysia.
Abstract | Pasteurellosis or fowl cholera (FC), is an economically-important poultry disease caused by Pasteurella multocida serotype A. In ducks, it is caused by Riemerella anatipestifer. Outbreaks of FC is frequently reported, suggestive of inefficacious vaccine. In this study, recombinant cells carrying OMP36 gene of Pasteurella multocida A:1 was prepared as a killed vaccine to test against pasteurellosis in chickens and ducks. A total of 325 chickens and 125 ducks were selected and divided to 4 groups based on the types of immunization. Groups 1, 2, 3, and 4 were administered with the recombinant vaccine, control E. coli without insert, commercial FC vaccine, and sterile PBS, respectively. A booster dose was administered after 2 weeks. Each group were then further divided to 4 subgroups based on birds and challenge strain. A challenge trial was conducted against P. multocida A:1, A:3, or A:1,3 for the chickens; and P. multocida A:1 and R. anatipestifer for the ducks. Clinical signs were observed and serum samples were collected weekly for determination of IgG antibody levels. Birds with severe clinical signs were euthanized for necropsy. Following immunization of chickens, IgG increased at week 1 and continued to increase after booster vaccination at week 2, and peaked at week 4 for both recombinant and commercial vaccines. The recombinant vaccine stimulated higher level of antibody in chickens. Following challenge, the recombinant vaccine provided excellent homologous (21/25; 84%) and cross-protection against P. multocida serotype A:1,3 (23/25; 92%) but provided low cross-protection against P. multocida serotype A:3 (11/25; 44%). In ducks, the recombinant vaccine provided moderate protection (14/25; 56%) compared to the excellent protection provided by the commercial vaccine (23/25; 92%). All euthanized birds showed typical clinical signs and gross pathology of FC. In conclusion, the recombinant vaccine is suitable against pasteurellosis in chicken, being more superior compared to the commercial vaccine. However, it provides poor protection against R. anatipestifer infection in ducks.
Keywords | Chickens, Ducks, OMP36, Pasteurella multocida, Riemerella anatipestifer
Received | August 23, 2019; Accepted | November 12, 2019; Published | November 26, 2019
*Correspondence | Annas Salleh, Department of Veterinary Laboratory Diagnosis, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Malaysia; Email: annas@upm.edu.my
Citation | Handijatno D, Ahmad N, Yusoff SM, Salleh A, Zamri-Saad M (2019). Efficacy of a recombinant vaccine against pasteurellosis in chickens and ducks. Adv. Anim. Vet. Sci. 7(12): 1134-1139.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.12.1134.1139
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Handijatno et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pasteurellosis, also known as fowl cholera (FC) in chickens is caused by Pasteurella multocida. All serotypes of P. multocida have been previously isolated from avian hosts (Christensen et al., 2008), except capsular types E and serotypes 8 and 13. The capsular type A has been observed to be the most frequently isolated from cases of severe FC (Christensen et al., 2008). In ducks, infections by P. multocida or Riemerella antipestifer (previously in the genus Pasteurella), or both are sometimes known as FC. The disease leads to great economic loss in poultry and duck industries, mainly due to the high mortality (Baksi et al., 2018). This high mortality is the result of acute septicaemia or chronic respiratory infection by these pathogens (Homchampa et al., 1997). Sulpha drugs, semisynthetic penicillin, tetracycline, and erythromycin are administered in food or drinking water to control the disease (Huang et al., 2009). However, the usage of antimicrobials in animal production raises global concern pertaining to incidence of anti-microbial resistance (Stella et al., 2018).
Immunisation has also been used to control FC particularly in layers and breeders. Currently, commercially-available vaccines are in the form of bacterin, and only induce immunity to homologous serotypes (Homchompa et al., 1997). On the other hand, the use of live vaccine confers immunity against heterologous serotypes, but may revert to cause infection (Christensen et al., 2008). Furthermore, anaphylactic shock and short duration of protection are among the limitations of immunization using bacterin (Gershwin, 2015; Dellagostin et al., 2017). Hence, it is necessary to design a safe vaccine capable of conferring protection against homologous and heterologous challenges.
Recent vaccine development uses the outer membrane protein (OMP), which is part of the bacterial cell wall considered as a potent candidate for vaccine (Poolperm et al., 2018; Anun, 2001). The vaccine is prepared as recombinant cells that carry selected gene to provide better protection against challenge by P. multocida (Ibrahim et al., 2000). The objective of this study was conducted to compare the efficacy of a recombinant cell carrying OPM36 gene of P. multocida A:1 against FC in chickens and ducks.
MATERIALS AND METHODS
Experimental Animals
A total of 325 Cobb chickens and 125 White Pekin ducks of 14 days old with no history of immunization against P. multocida and R. anatipestifer were selected. The chickens or the ducks were originated from one source. The birds were previously immunized against Marek’s, Newcastle, and Gumboro diseases (Prabakaran, 2003). At the animal facility, they were kept in pens and were provided with commercial feed and drinking water. They were acclimatized for a week, while oral swabs were collected the following week for screening of P. multocida. The experiment was started when P. multocida was absent from all birds at which the birds were at 28 days old. All animal experiment was approved by an ethical review committee.
Vaccines
Recombinant OMP 36 kDa gene of P. multocida serotype A:1 was previously prepared, inserted into pET 32 KL/LIC vector, and transformed into Escherichia coli BL21(DE3) strain before they were grown in Luria-Bertani broth with induction using isopropyl-β-D-thiogalactoside (IPTG). The culture was incubated at 26oC for 24h. The culture was harvested for determination of bacterial concentration (Pommerville, 2014). It was then washed with PBS, and killed using 0.5% formalin at 4oC for 18h. The culture was washed three times and re-suspended in PBS to a concentration of 3.0x108 cells/ml.
The control non-inserted vaccine was similarly prepared but without the OMP36 gene insert. Other control vaccine used for chickens was commercial alum-precipitated vaccine containing 3.0x108 cfu/ml of P. multocida A:1 (MVP, Malaysia). For ducks, a commercial alum-precipitated vaccine containing 3.0x108 cfu/ml of P. multocida serotype A:1 and R. anatipestifer (MVP, Malaysia) was used. Sterile PBS was used as negative control.
Bacterial Inoculums
Isolates of P. multocida serotypes A:1, A:3, A:1, 3 and R. anatipestifer from previous outbreaks of FC were obtained from stock cultures kept at -80°C. They were thawed at room temperature for 1h, and subsequently cultured onto blood agar and incubated at 37oC for 24h. Fifteen colonies were selected from each serotype and cultured in brain-heart infusion (BHI) broth, and incubated at 37oC for 24h. The culture was adjusted to a final bacterial concentration of 1.0x109 cfu/ml.
Experimental Design
Immunization and challenge
The chickens and ducks were divided to 4 groups based on the types of immunization. Group 1 were immunized with the recombinant vaccine, Group 2 were with E. coli without insert, Group 3 with commercial FC vaccine, and Group 4 with sterile PBS. The immunization was done at the dose of 0.5ml, intramuscular. Two weeks after the first immunization, booster dose of respective vaccine was administered with similar dose and route. Two weeks after the booster dose, the birds were then further divided into subgroups based on the challenge strains and were challenged with the inoculums prepared (Table 1).
Blood sampling and ELISA
Blood samples were collected via the brachial vein at weekly interval. The serum was subjected to determination of IgG level against P. multocida A:1 using ELISA as previously described, with minor modification (Sthitmatee et al., 2008; Solano et al., 1983). Briefly, microplates were coated with 50µl of antigen; 3.0x106 cfu/ml P. multocida serotype A:1 in carbonate-bicarbonate buffer (pH 9.6), and incubated at 4oC overnight. The plates were then washed, filled with 200µl of blocking buffer [PBS-0.5% Tween 20(PBS-T)-3% BSA] and incubated at 37oC for 1h. After washing, 50µl of diluted serum samples in blocking buffer (1:500) were added, and incubated at 37oC for 1 h. The plates were washed, and filled with 50µl of diluted goat anti-chicken IgG peroxidase conjugate (i-DNA, USA) in blocking buffer (1: 10,000) and incubated at 37oC for 1 h. Following washing, 100µl of TMB solution substrate buffer was added and incubated at 37oC for 30 min. The reaction was stopped by adding 2N sulphuric acid. Absorbance value was measured at 450 nm wavelength. The absorbance values were plotted to compare between different groups.
Table 1: Summary of the chicken and duck groupings, immunization, and challenge
| Groups |
Immunization1 |
Birds | Challenge strain |
| Group 1 | Recombinant | Chicken |
P. multocida A:1 |
| Chicken |
P. multocida A:3 |
||
| Chicken |
P. multocida A:1,3 |
||
| Duck |
P. multocida A:1 + R. anatipestifer |
||
| Group 2 |
E. coli no insert |
Chicken |
P. multocida A:1 |
| Chicken |
P. multocida A:3 |
||
| Chicken |
P. multocida A:1,3 |
||
| Duck |
P. multocida A:1 + R. anatipestifer |
||
| Group 3 | Commercial | Chicken |
P. multocida A:1 |
| Chicken |
P. multocida A:3 |
||
| Chicken |
P. multocida A:1,3 |
||
| Duck |
P. multocida A:1 + R. anatipestifer |
||
| Group 4 | PBS | Chicken |
P. multocida A:1 |
| Chicken |
P. multocida A:3 |
||
| Chicken |
P. multocida A:1,3 |
||
| Chicken | none | ||
| Duck |
P. multocida A:1 + R. anatipestifer |
||
| Duck | none | ||
| Group 5 | PBS | Chicken | none |
| Duck | none |
1Birds were immunized 2 weeks apart with respective vaccine (0.5ml, IM).
Clinical signs, survival rate, and post-mortem examination
All birds were observed for a period of 2 weeks. Birds with severe clinical signs of FC were euthanized by slaughtering (Grandin, 1994), and were considered succumbed to the infection. Any surviving birds were euthanized at the end of the experiment. Survival rate was calculated for each group. Immediately after euthanasia, liver, lung, pericardial fluid, heart and spleen samples were collected for bacterial isolation.
Bacterial isolation
The abovementioned samples were cultured on blood agar and incubated at 37oC for 24h. Pasteurella multocida and R. anatipestifer was identified using Gram stain and biochemical tests (Carter and Cole, 2012; Peter et al., 1996), and subsequent confirmation of P. multocida (Shivachandra et al. 2006) and R. anatipestifer (Kardos et al., 2006) were made with PCR.
Statistical analysis
Data of IgG antibody levels in the serum samples were tabulated and analyzed using One Way ANOVA and comparison of mean by Duncan test. Data on percentage of animal that died after challenge were tabulated and analyzed using Chi-square.
RESULTS
Survival rate in chickens
Chickens immunized with the recombinant vaccine showed 84% (21/25), 44% (11/25) and 92% (23/25) survival rates following challenged by P. multocida A:1, A:3 and A:1,3, respectively. The recombinant vaccine provided significantly (p<0.05) lower protection against P. multocida A:3 compared to P. multocida A:1 and A:1,3. In comparison, chickens immunized by the commercial vaccine showed significantly (p<0.05) lower level of protection at 64% (16/25) and 44% (11/25) against P. multocida A:1 and A:3, respectively but higher (p>0.05) level of protection at 80% (20/25) against P. multocida 1,3. The E. coli with no insert provided significantly (p<0.05) low homologous and heterologous protections at 40% (10/25), 32% (8/25) and 44% (11/25) against P. multocida A:1, A:3 and A:1,3, respectively. Only few chicken administered with PBS and challenged with P. multocida A:1, A:3, or A:1,3 survived the challenge trial (Table 2).
Survival rate in ducks
Following immunization with the recombinant vaccine, 56% (14/25) of the ducks were protected against a challenge by the combination of P. multocida A:1 and R. anatipestifer. In contrast, the commercial vaccine provided significantly (p<0.05) higher rate of protection at 92% (23/25). Immunization by E. coli failed to provide protection when significantly (p<0.05) low (20%; 5/25) number of the ducks survived. None of the duck administered with PBS and challenged with P. multocida A:1 and R. anatipestifer survived the challenge (Table 2).
Bacterial Isolation and Identification
The respective P. multocida was isolated from all organ samples of chickens, while R. anatipestifer was isolated from the lungs of ducks that succumbed to the challenge. All isolates of P. multocida were confirmed by PCR with the 1044 bp band while R. anatipestifer with the 700bp band (Figure 1). All organ samples from surviving chickens and ducks yielded no isolation of P. multocida or R. anatipestifer.
Table 2: Summary of immunization and survival rate after challenge trial in chickens and ducks.
| Groups |
Immunization1 |
Birds | Challenge strain | Survival rate % |
| Group 1 | Recombinant | Chicken |
P. multocida A:1 |
84a |
| Chicken |
P. multocida A:3 |
44b |
||
| Chicken |
P. multocida A:1,3 |
92a |
||
| Duck |
P. multocida A:1 + R. anatipestifer |
56b |
||
| Group 2 |
E. coli no insert |
Chicken |
P. multocida A:1 |
40c |
| Chicken |
P. multocida A:3 |
32c |
||
| Chicken |
P. multocida A:1,3 |
44b |
||
| Duck |
P. multocida A:1 + R. anatipestifer |
20c |
||
| Group 3 | Commercial | Chicken |
P. multocida A:1 |
64b |
| Chicken |
P. multocida A:3 |
44b |
||
| Chicken |
P. multocida A:1,3 |
80a |
||
| Duck |
P. multocida A:1 + R. anatipestifer |
92a |
||
| Group 4 | PBS | Chicken |
P. multocida A:1 |
8d |
| Chicken |
P. multocida A:3 |
4d |
||
| Chicken |
P. multocida A:1,3 |
16d |
||
| Chicken | none |
100a |
||
| Duck |
P. multocida A:1 + R. anatipestifer |
0d |
||
| Duck | none |
100a |
1Birds were immunized 2 weeks apart with respective vaccine (0.5ml, IM); a,b,c,dDifferent superscript letters signify significant (p<0.05) difference.
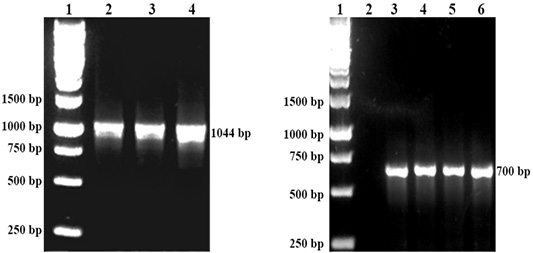
Figure 1: Left: DNA detection of P. multocida by PCR. Lane 1: Protein marker, Lane 2: P. multocida A:1, Lane 3: P. multocida A:3 Lane 4: P. multocida A:1,3. Right: DNA detection of R. anatipestifer by PCR. Lane 1 Protein marker. Lane 3,4,5 and 6: R. anatipestifer.
Serum IgG antibody response
Prior to immunization, there was significantly (p<0.05) low level of IgG in both chickens and ducks of all groups. However, following the first immunization, the IgG level increased significant (p<0.05) among the chickens and ducks immunized with the recombinant and commercial vaccines, but not following immunization with E. coli (Figure 2). The antibody levels increased until week 4 post-immunization, particularly after the administration of booster dose on week 2. Further increase of the IgG level was observed on week 5 following challenge but started to decrease on week 6.
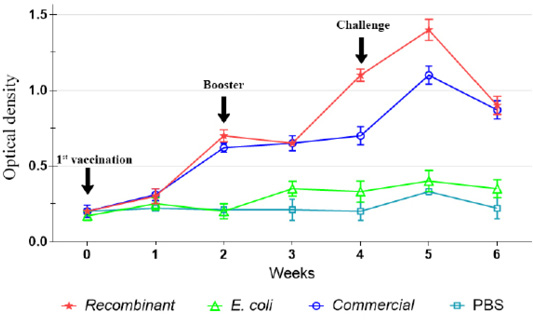
Figure 2: IgG antibody against P. multocida A:1 response in chickens during the immunization and challenge trial.
The IgG level was not significantly (p>0.05) increased following immunization with E. coli and in the control unimmunized groups. However, slight increase in the levels of IgG was observed on week 5 following challenge (Figures 2, 3).
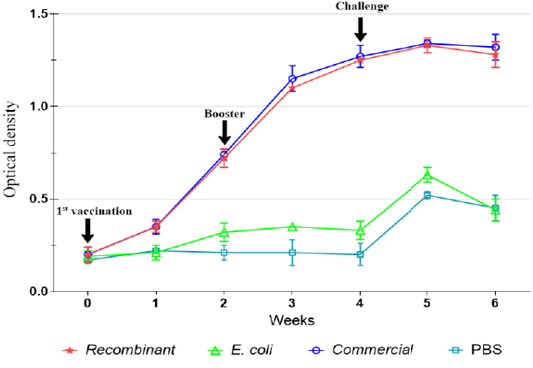
Figure 3: IgG antibody against P. multocida A:1 response in ducks during the immunization and challenge trial.
Clinical Signs and Gross Pathology
All challenged chickens and ducks that were euthanized due to severe clinical signs of FC had abnormal respiratory sounds, greenish diarrhea, weakness, and anorexia. These birds were observed with gross lesions including haemorrhage musculature, hepatic congestion (Figure 4), haemorrhagic trachea, air sacculitis, ventricular congestion, and hydropericardium.
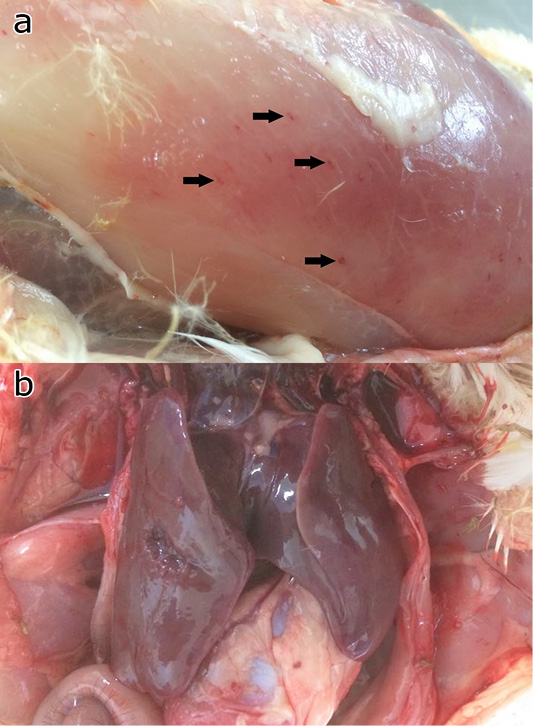
Figure 4: Gross pathology of the chicken and ducks showing petechia haemorrhage (arrows) of the pectoral muscle (a), and hepatic congestion (b).
DISCUSSION
Despite the availability of vaccines against FC, outbreaks were commonly reported globally, affecting both domestic and wild birds (Srinivasan et al., 2011; Hassan et al., 2017; Sellyei et al., 2017; Jaeger et al., 2018). This might be due to the poor usage of the currently available vaccines. This study investigates the attempts to use the OMP36 gene of P. multocida serotype A:1, expressed in E. coli strain BL21 through pET 32 plasmid as recombinant vaccine against FC in chicken and duck.
Following immunizations, the antibody titers increased significantly until week 4 following activation of memory cells (Dick and Avakian, 1991; Tizard, 2018). It seemed that both recombinant and commercial vaccines produced similar antibody pattern in chickens and ducks. Nevertheless, the antibody pattern following booster with the recombinant vaccine in chicken was significantly (p<0.05) higher than that of the commercial vaccine, possibly due to the superior antigenicity of recombinant OMP36 that was expressed more after induction with IPTG than the whole cell P. multocida in the commercial vaccine. On the other hand, antibody titers in ducks following exposures to recombinant and commercial vaccines showed no significant difference (p>0.05).
The impact of antibody response could be observed following challenge. The recombinant vaccine that produced higher antibody level was capable of providing better protection in chickens, particularly after challenged by P. multocida A:1 and A:1,3. However, the recombinant vaccine that did not significantly different from the commercial vaccine did poorly in ducks upon challenged by P. multocida A:1 and R. anatipestifer. The significant finding of this study is that the recombinant cells carrying OMP36 gene were capable of providing cross protection against homologous and heterologous P. multocida infection in chicken. Similar observations were previously reported (Chung et al., 2005; Sthitmatee et al., 2008), particularly due to the fact that vaccines made from bacteria that were cultured in vivo or in vitro under iron limited condition (Ibrahim et al., 2000; Kedrak and Opacka, 2003).
The low protection provided by the recombinant vaccine in the ducks suggested that the IgG antibody against P. multocida A:1 conferred were not capable of providing cross protection against infection by R. anatipestifer. Hence, further research should focus to improve this aspect. Detection and insertion of antigenic gene from R. anatipestifer into the existing recombinant cells to produce bivalent recombinant vaccine should be considered. However, this may be a challenge since previous study utilizing OMPA and P45 genes of R. anatipestifer had failed to provide effective protection in ducks against R. anatipestifer infection (Huang et al., 2002).
CONCLUSIONS
The study showed that the usage of recombinant vaccine derived from OMP 36 kDa gene of P. multocida serotype A:1 insert into pET 32 plasmid and E. coli strain BL21 as expression host cell able to provide high protection against homologous (P. multocida A:1) and heterologous P. multocida A:1,3, but provided moderate cross protection against heterologous P. multocida serotype A:3 in chickens. This is more superior compared to the tested commercial vaccine. In ducks, the recombinant vaccine able to provide partial protection against P. multocida A:1 and R. anatipestifer, but significantly lower than the tested commercial vaccine.
Authors Contributions
DH conducted the study, data analysis and writing of mauscript, NAA and AS involved in revision of manuscript, MZS and SMY designed and supervised the study.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
REFERENCES





