Advances in Animal and Veterinary Sciences
Research Article
Genotypic Characterization of Campylobacter Species Isolated from Livestock and Poultry and Evaluation of some Herbal Oil Antimicrobial Effect against Selected Campylobacter Species
Walid H. Hassan1, Ahmed E. Abdel-Ghany2, Samia I. Afifi3, Safaa H. Sedik4*
1Bacteriology, Mycology, and Immunology Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Hygiene, Zoonoses and Epidemiology Department, Faculty of Veterinary Medicine, Minia University, Minia, Egypt; 3Bacteriology Research Unit, Animal Health Research Institute, P.O. Box 264, Dokki, Gizza 12618, Egypt; 4Animal Health Research Institute, Beni-Suef Branch, Beni-Suef 62511, Egypt.
Abstract | Campylobacter food poisoning is underestimated in developing countries. This study aimed to investigate the prevalence and molecularly characterize Campylobacter spp. in cattle, sheep and poultry investigated in Beni-Suef Governorate, Egypt. Additionally, the MICs of some selected herbal oils on the isolated strains were studied. A total of 190 rectal swabs from cattle (n=85) and sheep (n=105) in addition to 200 samples from chickens (70 intestinal content and 130 cloacal swabs) were collected in the period October 2016 through January 2017. Bacteriological examination revealed that 37 (43.52%) out of 85 rectal samples obtained from cattle, as well as 37 out of 105 sheep samples (35.24%) harbored Campylobacter spp. In addition, 146 (73%) out of 200 examined chicken samples were bacteriologically positive. Analysis of the identified Campylobacter spp. revealed that the C. coli was more prevalent in cattle and sheep than C. jejuni (13.5 and 21.6%, respectively). In chickens, results showed also that C. coli was found in 15% of the tested samples, while C. jejuni failed detection. The results showed high prevalence rates of virulence genes in tested strains. The flaA gene as a Campylobacter pathogenic marker was detected in (100%) of analyzed strains. The Cdt toxin three subunits: CdtA, CdtB, and CdtC were also detected in all tested strains. Twenty highly virulent Campylobacter strains (14 C. coli and 6 C. jejuni) were exposed to herbal oils in order to determine the MIC. The results showed that MIC values of selected herbal oils against Campylobacter spp. were 500, 1000, 1500, and 2000 µg|ml for eugenol, cinnamon, allicin, and thyme, respectively. The studied essential oils appeared to be effective against the highly virulent local Campylobacter strains at low bactericidal concentrations, thus, emphasizing the significance of these oils as natural antimicrobial agents.
Keywords | Campylobacter spp., Virulence factors, Livestock, Poultry, Herbal oils
Received | July 15, 2019; Accepted | November 10, 2019; Published | November 26, 2019
*Correspondence | Safaa Sedik, Animal Health Research Institute, Beni-Suef Branch, Beni-Suef 62511, Egypt; Email: safaahamdy837@gmail.com
Citation | Hassan WH, Abdel-Ghany AE, Afifi SI, Sedik SH (2019). Genotypic characterization of Campylobacter species isolated from livestock and poultry and evaluation of some herbal oil antimicrobial effect against selected Campylobacter species. Adv. Anim. Vet. Sci. 7(12): 1083-1092.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.12.1083.1092
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Hassan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Campylobacter is the main zoonotic pathogen that causes foodborne enteritis with C. jejuni and C. coli being the most recovered species (Scallan et al., 2011). Campylobacter infections in humans are mainly associated with consumption of undercooked chickens, improperly cooked beef, pork and raw milk. Exposure to farm animals and untreated water are other transmission routes. The significant role of cattle and sheep as important reservoirs of Campylobacter has been proven through molecular epidemiological research (Kassa et al., 2006; Sahin et al., 2012). C. jejuni and C. coli are commonly carried as commensals in the intestine of these animals, thus posing a considerable problem for the disease controller. Frequent screening of these animals for fecal carriage is, therefore, an important step for avoiding spread of these pathogens to humans via the fecal-oral route.
Poultry, particularly broiler chickens, are as important as ruminants in the epidemiology of campylobacteriosis. Sources of infection for poultry include water, feed, and litter. The birds usually do not show any signs of disease, but bacteria from the intestines can contaminate carcass surfaces during evisceration in the slaughterhouse and subsequently may transmit to humans (Naglić et al., 2005). In fresh retail poultry products, Campylobacter occurs as a result of their colonization of the gastrointestinal tract of chickens during growth.
Chickens play a significant role as a source of human infections in the developed world, hence it was suggested that combating of colonization of Campylobacter spp. in avian host can decrease the incidence of campylobacteriosis (Meunier et al., 2016). Decreasing Campylobacter colonization of poultry by 2-log10 was found effective to reduce human infections by 30-fold. Therefore, research has been focused on understanding the colonization of poultry by Campylobacter, subsequently, even a small reduction could have an extremely positive impact on human health (Rosenquist et al., 2003).
The mechanism of Campylobacter pathogenicity has not been exactly explained yet, although several studies investigated the virulence factors involved in Campylobacter pathogenicity (adhesion, invasion, and cytotoxicity) (Khoshbakht et al., 2013). However, the wide genotypic and phenotypic diversity of the bacterial species belonging to the genus of Campylobacter made it difficult to conclude the pathogenicity dependent factors.
It was found that some C. jejuni strains cause serious illness, while other isolates are not pathogenic at all or induce mild symptoms in humans (Rivera-Amill et al., 2001). However, it is known that mechanisms of movement, chemotaxis, adhesion, transcytosis and host cell penetration, as well as toxin production, are necessary to induce campylobacteriosis in humans (Snelling et al., 2005). These molecular markers participate in adhesion and colonization (flaA), invasion (virB11), the wla gene which is probably involved in the expression of ganglioside mimics in Guillain-Barré syndrome and Cdt cluster (cdtA, cdtB, cdtC) which is responsible for toxin production (Wieczorek et al.,2011).
A major problem facing the efforts to control populations of food-borne pathogenic bacteria is the formation of protective biofilms produced by bacterial colonies. Campylobacter is 1000 times more resistant to antibiotics due to the presence such phenomenon. However, some essential oils are assumed able to solve such obstacle. For instance, garlic compounds were able to destroy the bacteria in just a fraction of the time taken by antibiotics like, erythromycin and ciprofloxacin (Xiaonan et al., 2012). Indeed, essential oils are used by some as ingredients in antimicrobial bioactive films to reduce the risks of food pathogens (Aminzare et al., 2017). Others proved their role in safety of meat products since they could delay spoilage and pathogenic microbial growth, retard the lipid oxidation and, consequently, increase shelf life of food product (Tajik et al., 2015; Aminzare et al., 2018). Hence, research on using essential oils for that purpose is increasing due to the demand for natural and safe food additives.
Feed manufacturers are targeting many natural antimicrobials from plant material as new kinds of consumer-accepted feed additives to achieve the required purpose (Navarro et al., 2015). Many of them have promising ability to reduce the bacteria both in vitro as well as in foods (Rattanachaikunsopon et al., 2010). In recent years numerous studies have shown that Campylobacter species are sensitive to a wide variety of plant-derived compounds such as carvacrol, cinnamaldehyde, eugenol, and thymol (Grill et al., 2012; Navarro et al., 2015).
Eugenol is extracted from many plants, specifically the S. aromaticum (clove), and its antimicrobial activity is based on the ability to permeabilize the cell membrane and interact with proteins. Such nonspecific permeabilization of the cytoplasmic membrane is evidenced with leakage of K+ and ATP from the cell by eugenol (Hemaiswarya and Doble, 2009). Another essential oil is cinnamon which has a notable antioxidant activity, especially depending on phenolic and polyphenolic compounds (Aminzare et al., 2015; Moarefian et al., 2013). Allicin, obtained from garlic, contains a wide range of thiosulphinates that are responsible for the antibacterial activity (Marks, 2014). Garlic contains diallyl sulfide, that effectively penetrates and dissolves the protective bio-layer, and ultimately destroys the bacteria (Xiaonan et al., 2012). In addition, thyme oils induce their antimicrobial activity by triggering structural and functional damages to the cell membrane. Carvacrol causes disintegration of outer membrane and ultimately release of lipopolysaccharides from Gram negative bacteria (La Storia et al., 2011).
The fact that foodborne campylobacteriosis is underestimated in developing countries including Egypt directed us to conduct the current study with the objectives to; determine the prevalence of the Campylobacter spp in in cattle, sheep and poultry samples, perform molecular characterization of randomly selected C. jejuni and C. coli local strains isolated from cattle, sheep, and chickens’ fecal samples targeting seven Campylobacter spp. virulence genes, and finally studying the minimum inhibitory concentrations (MICs) of some herbal oils (eugenol, cinnamon, allicin, and thyme oil) on Campylobacter spp. for use to control their populations in foods.
MATERIALS AND METHODS
Samples
A total of 190 rectal swabs from cattle (n=85) and sheep (n=105) in addition to 200 samples from chickens (70 intestinal content and 130 cloacal swabs) were collected through a 4-monthes period, October 2016 through January 2017. Rectal and cloacal swabs were collected using sterile cotton swabs. Cloacal swabs were collected from poultry just before slaughtering while in case of intestinal contents Ten grams from each sample were taken from the cecal part just after slaughtering through an opening by sterile scissor. All samples were transported to the laboratory in ice box as soon as possible for bacteriological examination.
Isolation and biochemical examination
The technique was applied as recommended by ISO 10272-1 (ISO, 2006), using Bolton selective enrichment broth (Oxoid, CM0983, UK) with Bolton Supplement (Oxoid, SR0183,UK) and laked horse blood (Oxoid, SR0048,UK), CCDA (charcoal, cefoperazone, desoxycholate agar) medium (Oxoid, CM0739,UK) with CCDA selective supplement (Oxoid, SR0155,UK) and Campy Gen TM microaerophilic sachet (Oxoid,UK) was used to provide microaerophilic conditions required for Campylobacter spp. growth.
Identification, genotyping and virulence genes detection in the isolated Campylobacter spp.
Bacterial DNA was extracted using QIAamp DNA mini kit (Qiagen, USA) according to the manufacturer instructions. Oligonucleotide primers (Metabion, Germany) that specifically amplify target Campylobacter spp. genes were used for molecular identification (Table 1). The PCR reactions were done in 25µl reactions using Taq DNA Polymerase Kit (Invitrogen, USA) according to the manufacturer instructions with using 20pmol concentration of each forward and reverse primers. The thermal profile consisted of initial denaturation at 94˚C for 5 min followed by 35 cycles of denaturation at 94˚C for 30 sec, annealing according to each primer pairs for 45 sec, extension at 72˚C for 45 sec, and the final extension at 72˚C for 10 min.
Evaluation of the effect of herbal oils on Campylobacter spp.
A total of 20 virulent Campylobacter spp. strains (14 C. coli and 6 C. jejuni) recovered from fecal samples of cattle, sheep and chickens as confirmed by PCR were exposed to herbal oils (eugenol, cinnamon, allicin, and thyme oil 10%) in order to determine the minimum inhibitory concentration (MIC). A bacterial suspension (1-2×108CFU∕ml, Raeisi et al., 2016) was prepared using physiological saline matching Mcfarland’s opacity tube 0.5 prepared by adding barium chloride to sulfuric acid. The mixture of the two chemicals forms a precipitate, that when in suspension is equivalent to approximately 1.5 x 108 colony forming units/ml. (Approximate formula per liter of processed water) consist of 1.0 g barium chloride and 10 ml concentrated sulfuric acid.
Allicin and eugenol were obtained from Wako Pure Chemical Industries, Ltd., Japan, while thyme and cinnamon were obtained from Chemical industries in Egypt. All herbal oils were prepared in a final concentration of 10% solution in Dimethyl sulfoxide (DMSO) as a solvent. All prepared herbal oils were sterilized by filtration using a Millipore cellulose filter membrane (0.45Ml pore diameter).
Agar plate dilution method
The antibacterial effect of the oils was performed by incorporating the solution directly into the melted Muller Hinton Agar medium with addition of 5% defibrinated sheep Blood (Christofilogiannis, 2001). Briefly, Muller Hinton Agar was prepared autoclaved at 121oC for 15 minutes, oils dissolved in DMSO 10% were added when the agar was cooled to 55oC with gentle shaking in order to distribute the oils in the medium evenly, then 5% defibrinated sheep blood was added with gentle shaking. The medium was poured in sterile plates and to determine the MIC, serial dilutions were prepared of oils solution beginning with the lowest concentration of 250 µg/mL then (500, 750, 1000, 1250, 1500, 2000 µg/mL). Control plates (oil-free) containing the medium 9ml plus 1 mL DMSO in distilled water (DW) instead of oils were also prepared as a negative control. The prepared plates were inoculated with each tested bacterial strain and then kept at 42° C under microaerophilic conditions. The growth of each bacterial strain was checked after 24 and 48 hours.
RESULTS AND DISCUSSION
Prevalence of Campylobacter in the examined samples
In the current study, a total of 190 rectal swabs from cattle (n=85) and sheep (n=105) and 200 samples from chickens (70 intestinal content and 130 cloacal swabs) were bacteriologically examined. The bacteriological examination of the tested samples revealed that out of 85 rectal samples obtained from cattle, 37 (43.52%) were positive. As shown in Table 2, out of 105 samples from sheep, 37 (35.24%) were positive. The results agreed with a prevalence rate reported by (Fernández et al., 2007) in Chile (39.3%) in case of samples obtained from cattle and (Kassa et al., 2006) 38% in case of sheep, near from some
Table 1: Oligonucleotide primers used for amplification of Campylobacter spp. virulence genes with annealing temperatures.
| Reference | Product (bp) | Annealing temperature | Primer sequence | ID | Organism | Gene |
| 55˚C | ATCAATTAACCTTCGAGCACCG | 23SR | ||||
| 462 bp |
58˚C
|
AAT TGA AAA TTG CTC CAA CTA TG | CeuE | C. coli | ceuE.F | |
| TGA TTT TAT TAT TTG TAG CAG CG | CeuE.R | |||||
| 589 bp |
55˚C
|
CTA TTT TAT TTT TGA GTG CTT GTG | mapA | C. jejuni | MapA.F | |
| GCT TTA TTT GCC ATT TGT TTT ATT A | MapA.R | |||||
| 855 bp |
53˚C
|
AATAAAAATGCTGATAAAACAGGTG | flaA | Campylobacter spp | flaA664 | |
| TACCGAACCAATGTCTGCTCTGATT | flaA1494 | |||||
| 494 bp |
TCTTGTGA GTTGCCTTACCCCTTTT |
VirB11 | VirB-232 | |||
| CCTGCGTGTCCTGTGTTATTTACCC | VirB-701 | |||||
| 672 bp |
46˚C
|
TTAAGAGCAAGATATGAAGGTG | wlaN | WlaN-DL39 | ||
| CCATTTGAATTGATATTTTTG | WlaN-DL41 | |||||
|
|
165 bp | 42˚C | GGAAATTGGATTTGGGGCTATACT | cdtA | GNW | |
| ATCAACAAGGATAATGGACAAT | IVH | |||||
| 495 bp | GTTAAAATCCCCTGCTATCAACCA | cdtB | VAT2 | |||
| GTTGGCACTTGGAATTTGCAAGGC | WMI-R | |||||
| 555 bp | TGGATGATAGCAGGGGATTTTAAC | cdtC | cWMI-F | |||
| TTGCACATAACCAAAAGGAAG | LPF-X | |||||
| 2212 bp | CTTTATGCATGTTCTTCTAAATTT | Cdt cluster | LYA-F | |||
| GTTAAAGGTGGGGTTATAATCATT | MII-R |
Table 2: Prevalence of Campylobacter spp. in chickens, cattle and sheep samples.
| Samples | Source animal species | |||||||||
| Chickens | Cattle | Sheep | ||||||||
| Intestinal contents (No.70) | Cloacal swabs(No.130) | Total | ||||||||
| No* | % | No | % | No | % | No | % | No | % | |
| Positive | 40 | 2 | 106 | 53 | 146 | 73 | 37 | 43.52 | 37 | 35.24 |
| Negative | 30 | 15 | 24 | 12 | 54 | 27 | 48 | 56.48 | 68 | 64.76 |
| Total | 70 | 35 | 130 | 65 | 200 | 100 | 85 | 100 | 105 | 100 |
* No; number of samples, %; the percentage of positive/total tested samples.
records all over the world (Fernández and Hitschfeld, 2009; Premarathne et al., 2017). This increase in the shedding of Campylobacter may refer to stress such as lambing, weaning, changing feed, animal movement or transport and environmental contamination originated from wildfowl excreta contaminating the animal water sources (Sutherland et al., 2009). Conversely, lower prevalence rates were reported by (Sutherland et al., 2009) and (Huber et al., 2015).
In chickens, the results revealed that 146 (73%) out of 200 examined samples were bacteriologically positive (Table 2) and these results are consistent with previous authors reported 76% (Bernadette et al., 2012) and 78% prevalence rates (Reddy and Zishiri, 2018). A higher prevalence of 87.2% (Wieczorek et al., 2012) and 96% was reported (Guessoum et al., 2016), while prevalence as low as was 60.2% (Simango, 2013) and 44.9% (Vaishnavi et al., 2015) were also reported. This variation in results among authors may be attributed to that shedding of Campylobacter spp. is of a seasonal pattern (Kassa et al., 2006). Generally, the high prevalence in this study coincides with the sampling period (through a 4-monthes period, October 2016 through January 2017) and/or to hygienic conditions under which tested animals were kept. This can be better explained considering the report of Chanyalew et al. (2013) who found that the occurrence of sheep fecal carriage by Campylobacter spp. were more prominent in the period August through December. Also, Englen et al. (2007) reported that the prevalence of Campylobacter in broiler flocks was significantly lower during January and February than that from September to December. Nevertheless, the high fecal carriage rates in the examined livestock and poultry increase their potential for being infective for other susceptible individuals and allowing Campylobacters to invade the human food chain as well.
Typing and molecular Identification of the isolated Campylobacter species
Analysis of Campylobacter species identified in the present study revealed that the C. coli is more prevalent than C. jejuni (13.5 and 21.6%, respectively) in case of cattle and sheep (Table 3). Similar findings were reported by (Sanad et al., 2011) who isolated C. coli (72.9%) more than C. jejuni (39.2%) from fecal samples of dairy cattle in USA, (Uaboi-Egbenni et al., 2011) who also recovered more C. coli (25.9%) than C. jejuni (3.4%) from fecal samples of non-diarrheic goat in South Africa and (Karikari et al., 2017) in Ghana and Niger. These findings confirm the assumption that that C. coli are more common in Africa (LaGier et al., 2004).
Table 3: Prevalence of different Campylobacter spp. in different animal species.
| Species | Source animal species | |||||
| Cattle | Sheep | Chickens | ||||
| No* | % | No | % | No | % | |
| C. lari | 29 | 78.4 | 25 | 67.56 | 121 | 83 |
| C. coli | 5 | 13.5 | 8 | 21.6 | 22 | 15 |
| C. jejuni | 3 | 8.1 | 4 | 10.8 | - | 0 |
| C. upsaliensis | - | 0 | - | 0 | 3 | 2 |
| Total | 37 | 100 | 37 | 100 | 146 | 100 |
* no; number, %; the percentage of positive/total tested samples.
In chickens, results showed also that C. coli was found in 15% of the tested samples, while C. jejuni was not detected (Table 3). These findings contrast with data obtained by other authors who in similar samples detected mostly C. jejuni (Simango, 2013) and (Vaishnavi et al., 2015). However, there are also some reports where C. coli was more predominant than C. jejuni in poultry meat (Lynch et al., 2011; Mackiw et al., 2012). The differences in C. jejuni and/or C. coli prevalence within Egypt and between countries may be due to several factors, including farming and slaughtering practices, geographical locations, or other risk factors such as the concentration of the farms in each location and their proximity to other livestock.
In this study, randomly selected five C. jejuni and C. coli strains isolated from cattle, sheep, and chickens’ fecal samples in Beni-Suef governorate were molecularly characterized. For this purpose, thirty typed Campylobacter species were subjected for typing PCR. As shown in Table 4, 52.6% of the tested Campylobacter species from chicken were confirmed by PCR, while 80% and 100% of sheep and cattle isolates, respectively were also confirmed. Seven virulence genes essential in the pathogenesis of Campylobacter spp. were identified by the PCR methodology. One of the best characterized Campylobacter pathogenic markers is the flaA gene which determines flagella formation, therefore bacteria motility and enterocyte colonization (Nuijten et al., 2000). Our results showed that flaA was detected in (100%) of analyzed strains (Table 5). Several reports obtained the same results (Wieczorek and Osek, 2011) and (Ghorbanalizadgan et al., 2018). All these results may suggest that the flaA gene product is necessary for bacterial colonization of animal alimentary tract and determines the stability of bacteria on the surface of contaminated poultry carcass (Wieczorek and Osek, 2011).
Table 4: Molecular detection and typing of selected Campylobacter spp.
| Source animal species | Tested isolates | Positive no. (%)* | C. coli ceuE | C. jejuni mapA |
| Chickens | 19 | 10 (52.6%) | 10 | 0 |
| Sheep | 6 | 6 (100%) | 3 | 3 |
| Cattle | 5 | 4 (80%) | 1 | 3 |
| Total | 30 | 20 (66.7%) | 14 | 6 |
* no; number, %; percentage of positive/total tested samples.
Toxins produced by Campylobacter might be another factor that possibly plays a role in disease development. CDT toxin is composed of three subunits: CdtA, CdtB, and CdtC are well-described toxin molecules produced by C. coli and C. jejuni which are necessary to induce a cytotoxic effect in vitro (Martinez et al., 2006). The three cdt genes subunits were detected in all tested strains (Table 5) in consistence with previous literature (Fernandes et al., 2010; Khoshbakht et al., 2013).
The high prevalence rates of virulence genes detected in tested isolates demonstrate their significant role in the pathogenicity of Campylobacter species (Reddy and Zishiery, 2018). The invasiveness markers of Campylobacter isolates, virB11 gene, localized in the pVir plasmid was not detected in any of the tested isolates possessed this gene. These results resemble those reported by other investigations (Feng et al., 2009; Khoshbakht et al., 2013). However, other studies showed a detection rate of 7-20% in Campylobacter spp. (Bang et al., 2003; Datta et al., 2003; Wieczorek and Osek, 2008).
Table 5: Virulence genes detection by multiplex PCR.
| Sample ID | Source | Tested isolates | flaA | VirB11 | wlaN | Cdt cluster | cdtA | cdtB | cdC | Cdt |
| 1 | Chickens | C. coli | + | - | - | + | + | + | + | + |
| 2 | Sheep | C. coli | + | - | - | + | + | + | + | + |
| 3 | C. jejuni | + | - | - | + | + | + | + | + | |
| 4 | Cattle | C. jejuni | + | - | - | + | + | + | + | + |
| 5 | C.coli | + | - | - | + | + | + | + | + |
These differences may be due to the plasmid nature of virB11 and the genetic variations of the isolates from various geographical areas (Carvalho et al., 2001).
The putative virulence wlaN gene is probably involved in the expression of ganglioside mimics in Guillain-Barré syndrome and heptosyltransfrase and β-1, 3-galactosyltransferase production (Linton et al., 2000; Datta et al., 2003) and results showed that none of the analyzed isolates carried this gene also.
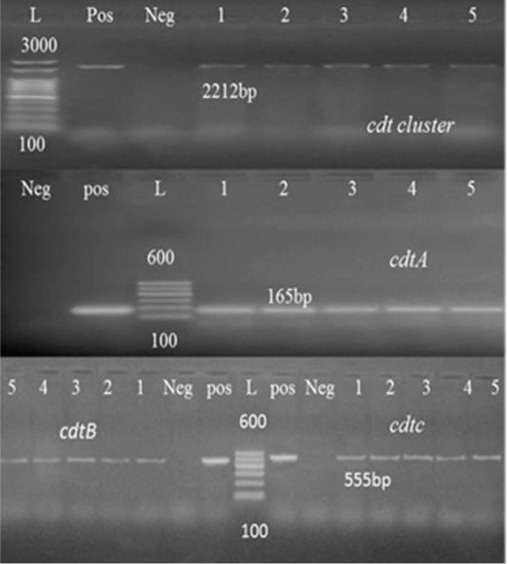
Figure 1:Agarose gel electrophoresis of cdt cluster, cdtA and cdtB PCR Products. Abbreviation: L: Ladder, Pos: Positive control, Neg: Negative control, lanes 1-5, tested Campylobacter Species samples.
Antibacterial effect of herbal oils on Campylobacter spp.
The antimicrobial properties of some natural herbal oils against Campylobacter spp. was investigated. A total of 20 confirmed highly virulent Campylobacter spp. strains (14 C. coli and 6 C. jejuni) were tested to determine the minimum inhibitory concentration (MIC). The results showed that MIC values were 500, 1000, 1500, and 2000 µg|ml for eugenol, cinnamon, allicin, and thyme, respectively. Primarily, the obtained results of EOs against Campylobacter Spp. confirms the previous concept that EOs are safe antimicrobial agents against a wide variety of pathogenic bacteria; Staphylococcus aureus, Escherichia coli, Listeria monocytogenes and Pseudomonas aeruginosa (Aminzare et al., 2017), L. monocytogenes (Tajik et al., 2015), Listeria monocytogenes and Staphylococcus aureus, Escherichia coli, and Salmonella typhimurium (Raeisi et al., 2016), and and Clostridium perfringens (Aminzare et al., 2018).
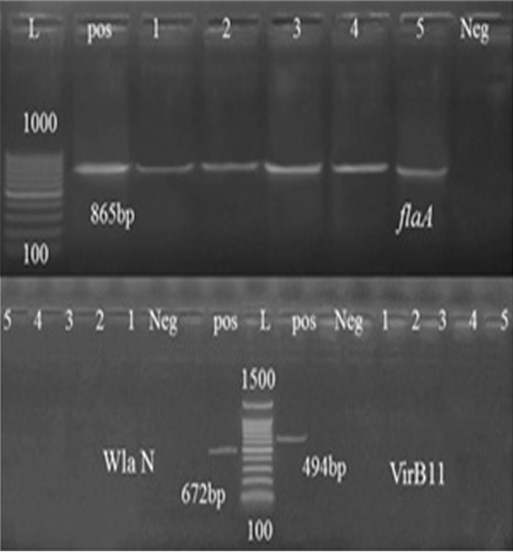
Figure 2: Agarose gel electrophoresis of FlaA, VirB11, and WlaN gene PCR. Abbreviation: L: Ladder, Pos: Positive control, Neg: Negative control, lanes 1-5, tested Campylobacter Species samples.
Among all the investigated herbal oils, eugenol was found as the most effective one against Campylobacter spp, meanwhile, thyme as 1500 µg|ml was able to inhibit the growth of 70% of the tested isolates. The results are in harmony with (Rattanachaikunsopon et al., 2010; Grill et al., 2012) who reported that the MIC of egunol oil when tested on C. jejuni was 0.12%. A comparable result of egunol (MIC 0.12%) was also demonstrated by (Grill et al., 2012). In the study of (Navarro et al., 2015), very low MIC values (below 0.02%) against Campylobacter was reached by eugenol and, by the same study, cinnamaldehyde showed middle range MIC values, between 0.020 and 0.05%.
Eugenol, the main component of clove EO, is thought by several authors to be responsible for the strong biological and antimicrobial properties of clove EO (Catherine et al., 2012). It has been proven to have effective anti-Campylobacter activity (Thanissery et al., 2014). In the present study, the MIC of cinnamon oil was 1000mgLml. Thyme which is main component of carvacol was found to be the least effective used oil as its MIC was 2000 mg/ml; at 1500 µg|ml it was able to inhibit the growth of 70% of the tested isolates. A MIC of 0.2% value was previously recorded by (Ravishankar et al., 2008) for carvacol. However, both cinnamon and carvacrol are phenolic compounds, with both hydrophobic and hydrophilic properties, interact with the lipid bilayer of the bacterial cytoplasmic membrane causing loss of its integrity (Zhu et al., 2016). More detectable efficacy of cinnamon, as a natural preservative agent in meat products, was previously documented in a combinational effect with grape seed extract (Aminzare et al., 2018).
Additionally, even subinhibitory concentrations of carvacrol are thought to block effectively the bacterial motility and invasion of eukaryotic cells which are considered key steps in C. jejuni infection, by interfering with flagella function without disturbing intracellular ATP levels. This concept broadens the spectrum of anti-microbial activity of carvacrol and supports the potential of the compound for use in novel infection prevention strategies (Van Alphen et al., 2012).
The current study showed also that allicin, the main component of garlic, induced its activity against bacteria at a MIC 1500 µg|ml. These results are well in line with those of (Rattanachaikunsopon et al., 2010) who studied the MIC of garlic and cinnamon oils on Campylobacter spp. and declared a MIC of 0.25 to 1 and 0.42 to 1 % respectively for the two oils. From the present study, it is possible to confirm the previous observations of (Kollanoor-Johny et al., 2010) who found that thymol, eugenol and carvacrol were all independently effective in significantly reducing Campylobacter concentrations. They remarked that Campylobacter reduction occurred after 15s of incubation in vitro, 20 mM (approximately 0.3%). After 8hr incubation, 10 mM concentrations of cinnamaldehyde, thymol, eugenol, or carvacrol were enough in decreasing C. jejuni by at least 5-log colony forming units (CFUs)/mL.
Variation in results of the antimicrobial results of plant-derived compounds and their MICs in different reports might be, in part, due to the use of different techniques to determine the MIC. Besides, purity and preservation status of the oil may play a role in these different findings (Navarro et al., 2015).
It is important to consider that eugenol, cinnamon, allicin, and thyme have a valuable activity against Quorum sensing (QS). In addition, essential oils might have a role as efflux pump inhibitors. QS is one mechanism in Campylobacter that is associated with biofilm formation. As QS communication has been linked to bacterial proliferation in foods and food spoilage, QS inhibition is a promising target to control Campylobacter and to ensure food safety (Nazzaro et al., 2013; Duarte et al., 2016). Essential oils have new anti-pathogenic drugs principle because of their anti-QS activity might be important in reducing virulence and pathogenicity of drug resistant bacteria in vivo. The mechanisms of action of the EOs include the degradation of the cell wall, damaging the cytoplasmic membrane, cytoplasm coagulation, damaging the membrane proteins, increased permeability leading to leakage of the cell contents, reducing the proton motive force, reducing the intracellular ATP pool via decreased ATP synthesis and augmented hydrolysis that is separate from the increased membrane permeability and reducing the membrane potential via increased membrane permeability (Nazzaro et al., 2013). On the other hand, some phenolic compounds present in several herbal oils act as efflux pump inhibitor, significantly reduce the expression of the CmeABC efflux pump and, therefore, could be used synergistically with antibiotics to inhibit Campylobacter spp. by impacting both antimicrobial influx and efflux (Oh and Jeon, 2015).
CONCLUSION
This study highlights the high prevalence rates of fecal carriage of Campylobacter spp. detected in cattle, sheep and poultry, which indicate the wide-spread of infection in Beni-Suef Governorate, Egypt. The potential role of these animals as significant reservoirs of infection to humans need to be further investigated. The molecular characterization of randomly selected C. jejuni and C. coli local strains revealed that C. coli were more predominant than C. jejuni. The high detection rates of virulence genes probably indicate their significant role in the pathogenicity of the isolated Campylobacter strains. Finally, the studied essential oils were found effective against the virulent Campylobacter strains at MIC of 500, 1000, 1500, and 2000 µg|ml for eugenol, cinnamon, allicin, and thyme, respectively. These data emphases the significance of these oils as natural antimicrobial agents.
ACKNOWLEDGMENTS
The authors would like to thank the Bacteriology, Mycology and Immunology Department staff at the Faculty of Veterinary Medicine, Beni-Suef University and staff in Animal Health Research Institute for providing technical help. Also, the authors would like to thank D. Ahmed Ali, Assistant Professor of poultry diseases at the Faculty of Veterinary Medicine, Beni-Suef University for his manuscript reviewing and editing.
Authors Contribution
All authors contributed equally in the planning of the study, drafting the manuscript. All of them approve the final version of the article.
conflict of interest
The authors declare there is no conflict of interest.
REFERENCES





