Advances in Animal and Veterinary Sciences
Research Article
Prevalence, Influential Factors and Oestrus Behaviour of Mares with Pneumovagina in a Tropical Region
Fuziaton Baharudin1*, Jasni Sabri2
1School of Biomedical Engineering and Health Science, Universiti Teknologi Malaysia, 81310 Johor Bahru, Malaysia; 2Department of Preclinical Study, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa City Campus, Locked Bag 36, 16100 Kota Bharu, Kelantan, Malaysia.
Abstract | This study determined the prevalence, the contributing factors, and the oestrus behaviour commonly observed in mares with pneumovagina in the tropical region. Pneumovagina is an anatomical conformational deformity due to inclined vulva that resulted in the vulva opening stretching beyond the limit set by the ischium of the pelvis. This condition compromised the vulva’s functional barrier in protecting the reproductive tract from contaminants. The current study involved 116 mares enrolled during clinical reproduction ambulatory work using purposive case-control cohort sampling method. Physical examinations were conducted to determine the Caslick’s index (CI) and body condition score (BCS). The oestrus behaviour was observed partly by the researcher. The recorded information was comprised of age, breed and reproductive history using a structured questionnaire responded by the owners. This study revealed that large breed mares with low BCS were those afflicted with the most severe pneumovagina. Brooding mares that had multiple pregnancies and first pregnancy before two years of age were more susceptible to severe pneumovagina at later stages of life. The mares with severe pneumovagina exhibited inconsistent oestrus intervals, yet strong stallion receptiveness. This behaviour mimicked the situation of irregular oestrogen secondary wave during the follicular phase with delayed LH surge at primary waves peak to facilitate ovulation. This situation implicates the erratic oestrus behaviour and poses challenges on reproduction management.
Keywords | Horse, Pneumovagina, Oestrus behaviour
Received | June 03, 2019; Accepted | August 19, 2019; Published | October 25, 2019
*Correspondence | Fuziaton Baharudin, School of Biomedical Engineering and Health Science, Universiti Teknologi Malaysia, 81310 Johor Bahru, Malaysia; Email: fuziaton@utm.my
Citation | Baharudin F, Sabri J (2019). Prevalence, influential factors and oestrus behaviour of mares with pneumovagina in a tropical region. Adv. Anim. Vet. Sci. 7(11): 937-943.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.11.937.943
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Bahadurin and Sabri. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pneumovagina is an abnormal perineal conformation commonly found in mares. This condition occurs when 20% of the vulva opening stretched beyond the ischium of the pelvis limit, making the function of the opposite two vulvar lips as the first barriers of the reproductive tract no longer available; thus, allowing intrusion of air, contaminants, and pathogens from the environment into the uterus (Newcombe, 2011; Goncagul et al., 2016). Mares with pneumovagina have been documented to be less fertile and have a lower pregnancy rate (Pascoe, 1979; Talluri et al., 2015).
The effective length and declination angle of the vulva in mares can be measured to provide an indicator called the Caslick’s index that determined the degree of perineal mal conformation (Pascoe, 1979). There are three types of pneumovagina identified; type-1 when the CI is <50 (10°), type-2 involved the CI of 50–100 (10°–20°), and type-3 with CI >150 (≥30°) (Davies-Morel, 2015). Type-1 mares do not require any corrective procedures and commonly have functional fertility status similar to normal mares, besides considered as having acceptable perineal conformation. Meanwhile, type-2 and type-3 mares are considered the severe types of pneumovagina (Davies-Morel, 2015) and require the Caslick’s vulvoplasty procedure to prevent reproductive tract contamination in ensuring adequate fertility (Pascoe, 2007; Talluri et al., 2015; Goncagul et al., 2016; Al-Fatlawy, 2017).
LeBlanc (2008) emphasised that any issues that cause low fertility in mares in stud farms are important and requires attention for corrective management. This is supported by financial reports of the equine industry, which revealed that if the failure to reproduce involve 63% of the mares in a farm over seven years of investment period due to increased age, multiple breeders or producing a low number of foals, negative financial return would occur (Bosh et al., 2010; Morris and Allen, 2010). Failure of reproduction in stud farms management is considered a critical issue and need to be addressed to ensure the investment of elite mares for optimal profitability (Ashbury, 1992). Pneumovagina is one of the factors affecting sound breeding performance in mares (Pycock, 2009; Pasolini et al., 2016); therefore, a physical examination of the reproductive system including evaluating pneumovagina should be conducted to predict the fertility potential of mares.
Vanniasingham et al. (1985) conducted a study on equine breeding management in Malaysia; however, the study lacked details on pneumovagina. Information about pneumovagina and the correlation between the signalments such as age, BCS, breed, type of activities, and vaginal stress due to repetitive pregnancies, to the development of pneumovagina in mares in Malaysia warrant investigation; therefore, is the objective of this study. The scope of this study includes quantifying a sample of mare population in Kelantan involved in breeding, sports, and recreational activities that are common in Malaysia; collection of data about the mares’ reproduction history and parameters from the farmers; physical examination of each mare in determining the types of pneumovagina; and field observation on oestrus behaviour during standing heat.
MATERIALS AND METHODS
Samples and Animal Selections
This study was carried out on 116 mares at the age ranging from 1 year to 20 years old of the common breeds available in Kelantan. The breeds of mares included in this study were the Thoroughbred, Thoroughbred cross, Arabs, Criollo, Quarter Paint, Appaloosa, Siam’s, Kelantan’s, and Sumbawa. The breeds were grouped based on the average size of the horses according to a general characteristic of every breed. The small breeds in this study were the ponies; Siam, Kelantan Cross, and Sumbawa ponies. The mediums breeds were the Quarter Paint, Appaloosa, Arabian, and Criollo, while the more substantial breeds were the Thoroughbred and Thoroughbred Cross. The activities of the mares included in this study were breeding, leisure, and sports. The selection of the mares was made using purposive sampling from a case-control cohort group from the field during reproduction ambulatory rotation involving the clients of Klinik Veterinar Universiti Malaysia Kelantan.
Survey of the Mares’ Reproductive Parameters
The information of the mares acquired using the questionnaire included identification, age, breed, colour, weight, height, fertility status, primary- and secondary function. Information on the reproduction history was obtained through the interviews with the farmers according to their records, which included the age of puberty, the age of first pregnancy, number of pregnancies, number of successful pregnancies, and any eventful situation during previous pregnancies such as foetal death and abortions.
Clinical Examination
Clinical evaluation was conducted to identify the BCS commonly applied on horses with the scoring from BCS 1 is considered thin or poor, BCS 3 is considered good, and BCS 5 is considered fat (Carroll and Huntington, 1988). The height and weight were measured using commercial nylon measuring tape. The tape was wrapped around the girth of the horse, directly behind the elbow, snugly in place, whereby the overlapping ends of the tape when the horse exhaled gave the weight measurement of the horse (Sendel, 1999).
The perineal conformation evaluation was measured by the angle of the vulva inclination (a) from the dorsal commissure of the vulva against an imaginary vertical line at the level of the pelvic brim using a plastic protector and a straight plastic ruler. Later, the length of the effective vulva opening (l) was measured from the ischium of the pelvis to the dorsal commissure using a soft plastic measuring tape. The angle and the length were then multiplied to gain the CI (Pascoe, 1979; 2007). The mares were grouped according to the CI commonly referred as the types of pneumovagina; type-1: CI<50 (10°), type-2: CI 50–100 (10° to 20°), and type-3: CI>150 (>30°) (Davies-Morel, 2015). The method of measurement is illustrated in Figure 1.
Oestrus Behaviour Assessment
In this study, the oestrus behaviour in the mares was tested using teaser stallions, and most mares were naturally covered. Positive oestrus behaviour is when there is winking of the vulva and vaginal discharge by the mares. The reaction of mares that stand firmly with the hind limb abducted to receive the stallion mounting during teasing was considered strong or intense oestrus behaviour. Meanwhile, mild oestrus was the condition when the mares were not standing firmly, refused to be mounted when teased by the stallion after the firmed standing.
The oestrus interval was measured by counting the days between two oestrus cycles starting from the last day of one heat exhibited by the mares up to the first day of the following heat. The interpretation of inconsistent oestrus behaviour was when the mares showed intermittent mild and robust oestrus behaviour during heat or no signs of oestrus at all with irregular standing heat pattern within oestrus. The oestrus behaviour of the mares was observed in the field. The oestrus behaviour, inter-oestrous interval and receptiveness of the stallion during standing heat were assessed using the guideline for breeding soundness examination in mares (Troedsson, 2014).
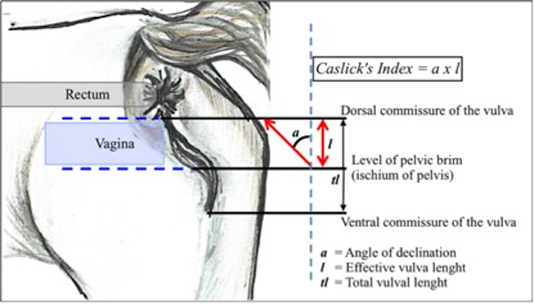
Figure 1: The angle of vulva inclination was measured from the pelvic brim to the dorsal commissure of the vulva. CI is determined by the outcome of the vulva inclination angle multiplied with the effective vulva length. The figure was adapted from a chapter in a textbook by Pascoe (2007)
Ethical Approval Procedure
This study has gained ethical approval from the Institutional Animal Care and Use Committee (IACUC) of Universiti Malaysia Kelantan on the 28th December 2018. The process of gaining approval included the submission of an application form with detailed procedures to be conducted on the animals and presentation of the research proposal to the IACUC. The reference number of the ethical approval is UMK/FPV/ACUE/PG/3/2019. All records and data were obtained with the owners’ consent for this study only.
Statistical Analysis
Chi-square test and correlation coefficient were applied in the data analysis in this study using IBM SPSS Statistics 24.0.0.0.
RESULTS
The Prevalence of Pneumovagina in Mares
Sixty-seven (58%) of the total 116 mares examined were found having vulva inclination; thus, pneumovagina and 49 (42%) of the mares were found negative. From the 67 mares with pneumovagina, 29 (43%) were having type-1, 19 (28%) were having type-2, and 19 (28%) were having type-3 as shown in Figure 2 (p= 0.001).
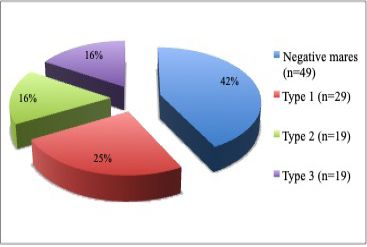
Figure 2: The pie chart shows the percentage of mares (n=116) with the types of pneumovagina. There were type-1 (n=29), type-2 (n=19), type-3 (n=19) and negative mares (n=49) without any vulva inclination.
Correlation Between Mares’ Activities and Pneumovagina
Mares having low impact activities in this study were involved in breeding, while mares with leisure and recreational activities showed a high incidence of pneumovagina. Out of the 67 positive mares, 22 (32%) were those involved primarily in breeding and leisure recreational activities. On the other hand, mares with highly intense activities were found to have a low incidence of pneumovagina with 21 mares (31%) were the polo ponies, 16 (23%) were the racing horses, and 8 (11%) were the endurance horses. These results have shown a positive correlation between low impact activities and the severity of pneumovagina (r2=0.1; p=0.009).
Correlation Between Bcs, Size, and Pneumovagina
The mares with poor BCS have shown severe types of pneumovagina compared to those with good BCS. Out of 8 mares from the total of 116 mares examined have BCS 1.5, whereby none of the mares has type-1 or negative pneumovagina, 3 (37%) were having type-2 pneumovagina, and 5 (63%) were having type-3. Meanwhile, out of 47 mares with BCS 2.0, 18 (38%) were negative, 15 (32%) were having type-1, 8 (2%) were having type-2, and 6 (1.3%) type-3. Furthermore, from 38 mares with BCS 2.5, 17 (45%) were negative of pneumovagina, 11 (29%) were having type-1, 6 (16%) were having type-2, and 4 (10%) were having type-3. Simultaneously, from 21 mares with BCS 3.0, 11 (52.3%) mares were negative of pneumovagina, 7 (33.3%) were having type-1, 3 (14.3%) were having type-2, and none of the mares had type-3. Only two mares in this study have BCS 3.5, and both of them (100%) did not have pneumovagina. The number of mares with increased severe types of pneumovagina significantly correlated to the decreased BCS (r2= – 0.1; p=0.046).
The small breed group comprised of 52 mares; whereby 26 (50%) did not have pneumovagina, 12 (23%) were having type-1, 6 (11.5%) were having type-2, and 8 (15.3%) were having type-3. As for the 38 mares grouped in the medium breed; 13 (34%) did not have pneumovagina, 12 (32%) were having type-1, 9 (24%) were having type-2, and 4 (10%) have type-3. Finally, for the 26 mares categorised as the larger breeds; 10 (38.5%) did not have pneumovagina, 5 (19%) were having type-1, 4 (15.5%) were type-2, and 7 (27%) were type-3. The correlation between the body size according to breeds and the types of pneumovagina were significant (r2=0.2; p= 0.001). The number of mares with severe types of pneumovagina increased with low BCS among the larger breed as illustrated in Figure 3.
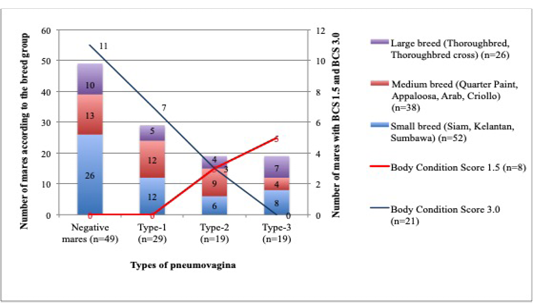
Figure 3: The mares were grouped according to the BCS and breeds for this analysis. A significant correlation between BCS1.5 (n=8), BCS3.0 (n=21) and the breed size of mares: small breed (n=52), medium breed (n=38), and the larger breed (n=26) with types of pneumovagina.
Correlation Between the Age of Mares with Pneumovagina
In this study, the age of mares did not show any significant difference (p= 0.082) or correlation coefficient (r2=0) with pneumovagina. The insignificance implicates that age was not the factor in the development of pneumovagina. Figure 4 shows equal distribution of the mares’ age and types of pneumovagina that can be explained as just a probability and not the influencing factor.
Influence of Pregnancies in Pneumovagina Development
In this study, 73 (63%) mares from the 116 mares studied had at least conceived once in their lifetime, and 43 (37%) of the mares were nulliparous. From the 73 mares that had conceived previously, 28 (38.5%) mares had at least conceived once, 23 (31.5%) had conceived twice, and 22 (30%) had more than three conceptions and considered as multiparous. From the total of 73 mares that had conceived previously, 27 (37%) of the mares had its first conception during the first 18 months old, 16 (22%) at the age between 18 to 36 months old, and 30 (41%) after 48 months old.
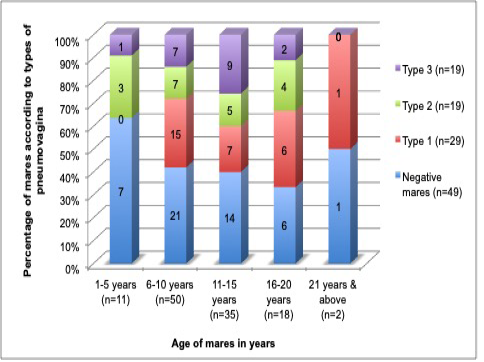
Figure 4: The age of the mares fairly distributed among the mares with all types of pneumovagina that indicate the age of the mares does not influence the types of pneumovagina. The group age ranged: 1 to 5 years old (n=11), 6 to 10 years old (n=50), 11 to 15 years old (n=35), 16 to 20 years old (n=18) and 21 years and above (n=2).
Among the 28 mares that had their first pregnancy within the first 18 months, 19 (68%) had type-2 and type-3 pneumovagina, while 3 (11%) have type-1 and 6 (21%) were negative of pneumovagina. Sixteen mares had their first conception between 18 to 36 months old; 4 (25%) had type-2, and type-3 pneumovagina, 6 (37.5%) have type-1, and other 6 (37.5%) were negative. However, from 30 mares that had their first conception after 48 months old, only 3 (10%) have type-2, and type-3 pneumovagina, 9 (30%) have type-1, and 18 (60%) were negative. Figure 5 shows the percentage of multiparous mares with more than three pregnancies and mares that had their first pregnancy within 18 months old with significant negative correlations.
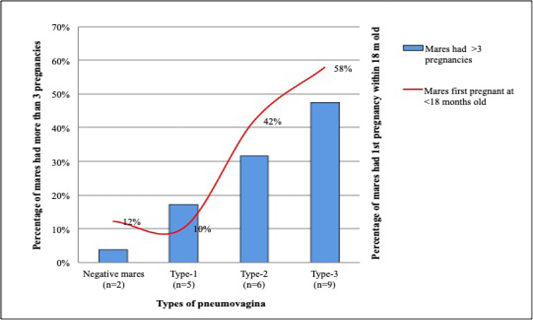
Figure 5: Mares that had its’ first pregnancy at early puberty showed higher cases of type-2 and type-3 pneumovagina. This trend is similar to mares that had more than three pregnancies.
Oestrus Behaviour of the Mares
Out of the 116 studied mares, 19 have type-3 pneumovagina with 10 (53%) mares showing inconsistent oestrus interval or no oestrus signs and 9 (47%) were having regular oestrus interval. The percentage of inconsistent oestrus interval reduced to 37% (n=7) in type-2 pneumovaginal mares (n=19), and increased number of mares with regular oestrus interval to 12 (63%). From 29 mares with type-1 pneumovagina, 7 (24%) showed inconsistent oestrus interval while 22 (76%) were normal. Furthermore, out of 49 negative mares, 10 (20%) mares showed inconsistent oestrus interval or no oestrus signs, while 39 (80%) showed normal oestrus. The result shows that mares with severe types of pneumovagina have higher chances to have inconsistent oestrus interval compared to the mares with negative pneumovagina, while the acceptable type of pneumovagina showed a higher percentage of having regular oestrus interval (r2=-0.32, p= 0.049).
Concurrently, mares with severe types of pneumovagina showed an intense standing heat by showing strong stallion reception during the teasing. A total of 19 mares with type-3 and 19 mares with type-2 pneumovagina have shown strong reception toward stallion during the standing heat that was extended up to 3 days. The number of mares with strong stallion reception was reduced to 75% (n=22) in type-1 pneumovagina mares and reduced further 50% (n=24) of 49 negative mares. As depicted in Figure 6, mares with inconsistent oestrus interval concurrent with the mares’ reception strength during standing heat according to the types of pneumovagina. The inconsistent oestrus behaviour and the types of pneumovagina were moderately correlated (r2=0.2, p= 0.5).
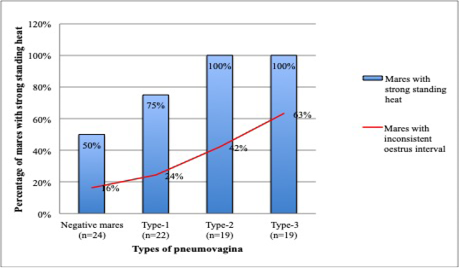
Figure 6: There are 38 mares with type-2 (n=19) and type-3 (n=19) pneumovaginal showed inconsistent oestrus interval or no signs of oestrus. All (n=38) severe types of mares showed strong stallion reception during standing heat. Only 75% (n=22) of the mares with type-1 and 50% (n=24) with negative pneumovaginal showed strong standing heat during the teasing by the stallion.
DISCUSSION
This study reports on the prevalence of pneumovagina among mares in Kelantan, Malaysia. The outcome of this study revealed that more than half (58%, n=67) of the 116 mares were suffering from pneumovagina. Among the positive mares, 43.3% (n=29) have the acceptable type-1 pneumovagina, and 56.6% (n=38) have severe type-2 and type-3. Based on this finding, this study concludes that pneumovagina is common among mares, with a high number of mares having severe type-2 and type-3.
Larger breed horses with poor BCS were likely to have severe pneumovagina compared to the smaller breed mares. This outcome agrees with the study conducted by Goddard and Allen (1989) who claimed that larger breed horses have lower intravaginal pressure and high uterine pressure that allowed air to enter through the vagina during physical movements of the internal organs or contraction of the abdominal muscle. The gradient of intra-abdominal pressure allows air from the external to enter the vagina and the uterus as studied by Newcombe (2011). This outcome is concurrent with the BCS evaluation, which implicated a high number of mares with poor BCS have severe types of pneumovagina compared to those with good BCS. This outcome supports the study conducted by Goddard and Allen (1989) that concluded mares with poor BCS have lesser abdominal fats surrounding the reproductive organ that affects the intravaginal pressure, which implicates the development of shortening and angulation of the vulva that signifies pneumovagina.
The mares involved in highly intense athletic activities such as racing, endurance, and polo have a lesser degree of pneumovagina compared to the mares that solely involved in breeding or light recreational activities. Pascoe (1979) reported high numbers of retired racing horses-turned-breeding mares to have pneumovagina, so as among endurance horses as reported by Ali et al. (2014).
This study did not show the significance or correlation between age of the mares and pneumovagina (p>0.05). The even distribution of mares categorised by the types of pneumovagina and the different age groups were in contrary to the previous studies that claimed pneumovagina developed due to old age (Pascoe, 1979; Al-Fatlawy, 2017). On the other hand, this study shows that multiparous mares have high numbers of severe pneumovagina compared to the nulliparous or the primiparous regardless of age. This study also implicated that younger multiparous mares have severe pneumovagina compared to older nulliparous mares. This study concludes that the age of mares is not the only contributing factor to the development of the severe types of pneumovagina, where other contributing factors such as multiple pregnancies should also be considered. These findings support the study conducted by Carnavale and Ginther (1992), who reported that older mares have lesser uterine elasticity that caused changes in the vaginal fibrotic structure.
The primiparous mares within 18 months old showed a significantly high incidence of pneumovagina during the later stage of life. The positive correlation (r2 = 0.11) between the age of primiparous and types of pneumovagina implicated that early exposure to reproductive hormones, especially diethylstilboestrol that was widely used in mares for effective reproductive management and commonly used among the farmers in Kelantan was among the contributing factors responsible for pneumovagina. Current findings are in accordance with Bern et al. (1975) that proved the drug had caused morphological alteration of the reproductive tract in older mice administered with the hormones during the neonate phase. Another study in human by Zong et al. (2010) also proved that the effect of synthetic hormones therapy in postmenopausal women reduced vaginal elastin, causing vaginal prolapse.
Mares with severe pneumovagina showed inconsistent oestrus intervals or did not show any oestrus cycle at all. On the other hand, mares with acceptable pneumovagina have consistent interovulation intervals between 14 to 28 days, which is considered within the normal range between 13 to 34 days, as recorded in a study conducted at a sub-tropical country (El-Wishy et al., 1990; Ali et al., 2014). Irregularity of the oestrus interval was commonly observed in mares with endometritis and pyometra in the study conducted by Al-Fatlawy (2017).
However, contradictory to the irregular oestrus interval, mares with the severe types of pneumovagina exhibited intense standing heat and were very receptive to stallion compared to only half of the negative mares and those that have type-1 pneumovagina. Normal mares have a standing heat that lasts for three days during the peak of the breeding season (Christensen, 2008). This irregular oestrus behaviour of the type-2 and type-3 pneumovaginal mares mimicked the situation when oestrogen peaked to the maximum during the primary wave but instilled a delay in the luteinising hormones surge to allow ovulation to take place (McCue, 2007); thus, causing the extension and intense standing heat.
Retrospectively, mares with the severe types of pneumovagina have lower pregnancy rate compared to those without pneumovagina or those with the acceptable type (Talluri et al., 2015; Al-Fatlawy, 2017). This study implicates that the inconsistent oestrus interval and extended standing heat of the mares with severe pneumovagina have caused ineffective and inefficient reproduction management that lead to the failure of conception.
The above findings warrant further investigation on the reproductive endocrine of the mares during the oestrus cycle. The extended standing heat in these mares has caused the farmers to naturally cover the mares twice to ensure pregnancy to take place. A further study to justify the reasons behind the lower number of pneumovagina incidence in high impact horse athlete could be conducted.
ACKNOWLEDGEMENTS
Heartfelt gratitude goes to Sarah Alia Mohd Radzi for her assistance in the data collection, processing, and spreadsheet establishment. The Ministry of Higher Education Malaysia funded this study through the Fundamental Research Grant Scheme (FRGS-1/2017) award (Reference no.: R/FRGS/A06.00/ 01254A/002/2017/000428).
CONFLICT OF INTEREST
There was no conflict of interest in this study declared by any of the authors.
AUTHORS CONTRIBUTION
Fuziaton Baharudin developed the theoretical formalism, performed the analytic calculations and the numerical simulations. Both Fuziaton Baharudin and Jasni Sabri contributed to the final version of the manuscript.
REFERENCES





