Advances in Animal and Veterinary Sciences
Research Article
Reindeer Parasites of the Khanty-Mansi Autonomous Region–Yugra (Russia): Feces Study
Anna Nikolaevna Siben1*, Vladimir Nikolaevich Domatsky1, Olga Alexandrovna Fiodorova1, Andrey Vasilyevich Klyatsky2
1All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology, Branch of Federal State Instution Federal Research Centre Tyumen Scientific Centre of Siberian Branch of the Russian Academy of Sciences, Institutskaya Street, 2, Tyumen, 625041, Russia
2Yugra State University, Institute of Oil and Gas, Chekhova Street, 16, Khanty-Mansiysk, 628012, Russia
Abstract | Reindeer breeding is the main focus of animal husbandry in the Far North. Reindeer helminthiases are widespread in natural habitats. This study was conducted at reindeer farms of various forms of ownership in the Berezovsky, Surgut, Beloyarsky and Nizhnevartovsky districts of the Khanty-Mansi Autonomous Region–Yugra and the Laboratory of Animal Myiases of the All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology of the Tyumen Scientific Centre of the Siberian Branch of the Russian Academy of Sciences. Faecal samples were taken individual animal (n=346) during 2017. Studies were conducted using the Fulleborn method and ether-acetic sedimentation. The infection rate in the region, on average was: Trichostrongylus sp.–31.8%, Trichocecpalus sp.–8.4%, Nematodirus sp.–12.4%, Dictyocaulus sp.–22.5%, Moniezia–sp.13.0%, Thysaniezia sp.–1.4%, Paramphistomidae–3.5%, Eimeria sp.–28.3%. Use of pluripotential anthelminthic drugs, which are effective against nematodoses, cestodiases and distomiases of animals, is recommended in problem farms of the region. A detailed study of the parasite species composition with the focus on parasites transmitted by blood-sucking insects is required in future research.
Keywords | Reindeer, Helminths, Nematodes, Moniezia, Paramphistomata, Khanty-Mansi Autonomous Region– Ugra (Russia)
Received | June 12, 2019; Accepted | August 30, 2019; Published | October 15, 2019
*Correspondence | Anna Nikolaevna Siben, All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology, Branch of Federal State Instution Federal Research Centre Tyumen Scientific Centre of Siberian Branch of the Russian Academy of Sciences, Institutskaya Street, 2, Tyumen, 625041, Russia; Email: jroschewitsch@mail.ru
Citation | Siben AN, VN Domatsky, OA Fiodorova, AV Klyatsky (2019). Reindeer parasites of the Khanty-Mansi autonomous Region–Yugra (Russia): feces study. Adv. Anim. Vet. Sci. 7(s1): 45-49.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s1.45.49
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Siben et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Reindeer breeding is the main focus of animal husbandry in the Far North. In addition to the direct production of various types of products, northern reindeer breeding has important social value, as it facilitates the maintenance of the traditional lifestyle of the indigenous peoples of the Far North. A number of factors hinder economically successful reindeer breeding including the reduction of grazing areas and the decrease in pasture productivity, as well as the incidence of animal diseases of various etiologies. The most negative impact is caused by the infection of reindeer by agents of infectious and invasive diseases. The studies of the diversity of reindeer parasites are devoted to the determination of species diversity and confirmation of the species validity (Khrustalev, 2012; Haukisalmi et al., 2018), the degree of parasites’ influence on the host organism, and the development of treatment modalities. According to Mitskevich (1967), reindeer carried 62 species of helminths. The data of modern researchers show that this species is infected with: Ostertagia gruehneri and Setaria tundra (Okulewicz, 2017) in northern and eastern Europe, Elaphostrongylus rangiferi in northern Europe (Halvorsen, 2012); Setaria tundra, Onchocerca spp. and Rumenfilaria andersoni in Finland (Laaksonen et al., 2017), Nematodirella longissimespiculata, Strong spp, Eimeria rangiferis in Greenland (Skírnisson and Cuyler, 2015). In Russia, Shalaeva (2017) found Cysticercus tenuicollis, Taenia parenchimatosa, Taenia krabbei [Cysticercus tarandi], Moniezia expansa, Avitellina arctica, Dicyocaulus eckerti, D. hadweni, Nematodirus tarandi and Nematodirella longissimespiculata in wild reindeers of West Taimyr. Ostertagia spp., Nematodirus spp., Moniezia spp. were found in domesticated reindeer of Taimyr (Zhelyakova et al., 2016). In the Yamalo-Nenets Autonomous Region, reindeer are infected with Moniezia baeri, M. rangiferina, M. benedeni, M. expansa, Avitellina arctica, Thysaniezia giardi (Siben et al., 2015), Nematodirus spp., M. digitatus, Trichostrongylus spp., Elaphostrongylus rangiferi, Parabronema skrjabini (Loginova et al., 2018). According to Pochepko (2014), in the Murmansk region, reindeer infection rate by Paramphistomidae in some farms reaches 100%. The high parasite infection rate in reindeer is due to the wide adaptive capabilities of helminths to survive in the harsh conditions of the Far North. Such adaptations include the inhibition ability, which is described in detail using the example of Ostertagia gruehneri in the paper by Hoar et al. (2012), as well as the ability to retain infectivity at low temperatures, as exemplified by Marshallagia marshalli (Carlsson et al., 2012; Carlsson et al., 2013) and Trichostrongylidae in reindeer in Svalbard (Halvorsen et al., 1999). Parasites have multiple effects on the animal body. In the paper by Carlsson et al. (2016), it is noted that the infection of Ostertagia gruehneri in reindeer leads to a decrease in the content of glucocorticoid metabolites in feces compared to uninfected animals. In another paper by Carlsson et al. (2018), the study of Marshallagia marshalli showed that the parasite had no negative effects on the reproductive abilities of female reindeer in Svalbard. However, according to Arneberg et al. (1996), parasitic gastrointestinal nematodes significantly reduce the average food intake, which may lead to a decrease in productivity. It is important to state that the pathogenic effect of the parasite on the host organism is mainly connected with its localization, and the absence of a pronounced effect from a nematode in the gastrointestinal tract is incomparable with the effect of the Setaria nematode. According to Laaksonen et al. (2007) and Haider et al. (2018), Setaria tundra causes peritonitis and damage to internal organs, which lead to significant economic losses.
Therefore, the aim of our research was to study the infection rate of parasites in reindeer in the Khanty-Mansi Autonomous Region–Yugra (Russia) and to ascertain future control strategies.
The studies were conducted from 23.10.2017 to 24.11.2017 on the basis of reindeer farms of various forms of ownership in the Berezovsky, Surgut, Beloyarsky and Nizhnevartovsky districts of the Khanty-Mansi Autonomous Region–Yugra and the Laboratory of Animal Myiases of the All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology of the Tyumen Scientific Centre of the Siberian Branch of the Russian Academy of Sciences, Tyumen (Table 1). The sampling of pathological material for the study was carried out in the fall-winter period of 2017. The samples of reindeer feces were used for the study, which were studied using the Fulleborn method and ether-acetic sedimentation.
Table 1: The number of studied samples by districts (2017).
| No. | Study region | Samples studied |
| 1. | Nizhnevartovsky | 60 |
| 2. | Surgut | 60 |
| 3. | Beloyarsky | 88 |
| 4. | Berezovsky | 138 |
|
Total: |
346 | |
Fulleborn method. Feces (5 g) were mixed with a saturated solution of sodium chloride (150-200 ml) in a mortar. Filtered the feces solution through gauze or a sieve, and poured into a glass cylindrical vessel and leave for 15-20 minutes. Eggs of parasitic worms, which have less volume weight, floated to the surface of the liquid. This method is effective for detecting the eggs of most nematodes and cestodes (Kotelnikov, 1974).
Ether-acetic sedimentation of helminth eggs. Sequential processing of feces samples using 10% aqueous solution of acetic acid and ether. Helminth eggs, especially trematode eggs, could be easily detected (Borzunov et al., 2004). We used a slightly modified method (the scheme is presented below).
Pour 8 ml of a 10% acetic acid solution into the centrifuge tube and add 1 g of feces. Stir thoroughly, and then filter through a funnel with two layers of gauze into another centrifuge tube. Add 2 ml of ethyl ether to the resulting emulsion (to get 10 ml of solution). Close the tubes with a stopper and thoroughly shake for 15 seconds, then leave for 10-15 minutes. Centrifuge for three minutes at 1500 rpm. Separate the resulting fecal mass from the wall of the tube and discharge the supernatant in one motion. Leave approximately 1 ml in the tube. Transfer the precipitate onto a glass slide and use for microscopic examination.
The distribution of reindeer helminths was estimated by calculating the index of the extensiveness of infection, which shows the ratio of the number of infected animals to the abundance of the surveyed population, expressed as a percentage. Helminth species were determined by eggs and larvae using the field guide by Mitskevich (1967).
Table 2: Infection rate of reindeer by parasites by districts (2017).
| No | Region of study | Extensiveness of infection, % | |||||||
|
Trichostrongylus sp. |
Trichocecpalus sp |
Nematodirus sp |
Dictyocaulus sp |
Moniezia sp. |
Thysaniezia sp. |
Paramphistomidae |
Eimeria sp |
||
| 1 | Nizhnevartovsky | 21.7 | 1.7 | - | - | - | 8.3 | - | 71.7 |
| 2 | Surgut | 48.3 | 33.3 | 21.6 | 21.7 | 13.3 | - | - | 38.3 |
| 3 | Beloyarsky | 27.3 | 9.1 | - | 50.0 | 4.5 | - | 3.5 | 15.9 |
| 4 | Berezovsky | 31.9 | - | 21.7 | 15.2 | 23.9 | - | 2.9 | 13.0 |
|
Total: |
31.8 | 8.4 | 12.4 | 22.5 | 13.0 | 1.4 | 3.5 | 28.3 | |
RESULTS
Analysis of the results of parasitological studies of domestic reindeer feces samples at the farms of the Nizhnevartovsky, Surgut, Beloyarsky and Berezovsky districts of the Khanty-Mansi Autonomous Region–Yugra indicates parasite infection of animals by nematodes, cestodes, trematodes and coccidia (Table 2).
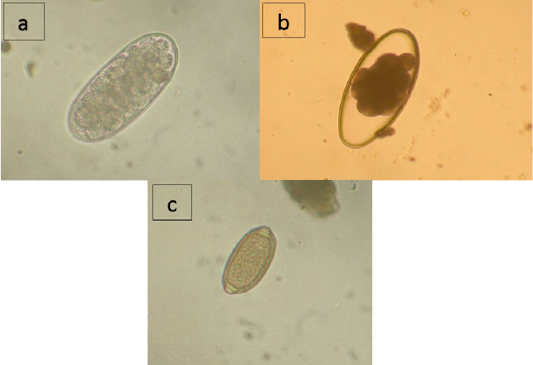
As shown by the data presented in Table 2, domestic reindeer of the region are infected with nematodes from families Trichostrongylidae (Trichostrongylus sp., Nematodirus sp.) and Trichocecpalata (Trichocecpalus sp.) that live in the gastrointestinal tract, and family Dictyocaulidae (Dictyocaulus sp.) that live in the lungs. According to the studies of the feces samples, Trichostrongylidae eggs were found in reindeer in all districts of the region, and the infection extensiveness (IE) varied from 21.3% in the Nizhnevartovsky district to 48.3% in the Surgut district (Figure 1a). Nematodirus infection was detected in the Surgut district with IE of 21.6% and the Berezovsky district with IE of 21.7% (Figure 1b). Trichocephalus eggs were found in feces samples of reindeer from the Nizhnevartovsky (IE = 1.7%), Surgut (IE = 33.3%), and the Beloyarsky (IE = 9.1%) districts (Figure 1c). Larvae of Dictyocaulidae were found in animals in all studied districts except Nizhnevartovsky, in the Beloyarsky district, IE was 50.0%, in the Berezovsky district, IE was 15.2%, in the Surgut district, IE was 21.7%.
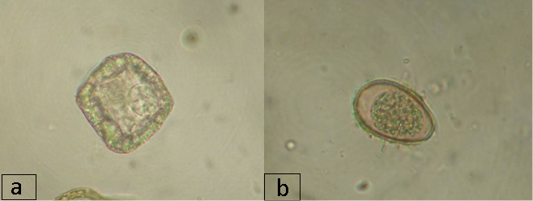
Imaginal cestodiases in reindeer in the region during the study period were caused by representatives of the families Avitellinidae (Thysaniezia sp.) and Anoplocephalidae (Moniezia sp.). Thysaniezia eggs were found in the reindeer feces only at the farms of the Nizhnevartovsky district (IE was 8.3%). Moniezia infection was observed in all districts except Nizhnevartovsky, in the Berezovsky district, IE was the highest and reached 23.9%, in the Surgut and Beloyarsky districts, IE was 13.3% and 4.5%, respectively (Figure 2a). Trematode infection in reindeer in the region was caused by a representative of the Paramphistomidae suborder and was recorded at the farms of the Beloyarsky and Berezovsky districts, where IE was 3.5% and 2.9%, respectively. IE was not high, which may be due to the season of the year or the special climatic and geographical features of the region or dehelminthisation activities.
In addition to the eggs and larvae of the above-mentioned parasitic helminths that live in the gastrointestinal tract and lungs, coccidian oocysts were found in the animal feces (family Eimeriidae, Figure 2b). The highest infection rate by Coccidia in reindeer was found at the farms of the Nizhnevartovsky district with IE of 71.7%, IE in the Beloyarsky, Berezovsky, and Surgut districts was 15.9%, 13.0% and 38.3%, respectively.
DISCUSSION
Reindeer parasites, the diagnosis of which is based on the examination of feces samples, are nematodes, cestodes, trematodes and protozoa. Nematode parasitism in animals is the most thoroughly studied subject (Okulewicz, 2017; Halvorsen, 2012; Laaksonen et al., 2017; Hoar et al., 2012; Carlsson et al., 2012; Carlsson, 2013). The purpose of our research was to study the parasite infection rate in reindeer in the Khanty-Mansi Autonomous Region– Yugra (Russia) based on the results of the feces samples study. Scatoscopy studies revealed parasite infections of Trichostrongylus sp. with IE of 31.8%, Trichocecpalus sp. with IE of 8.4%, Nematodirus sp. with IE of 12.4%, Dictyocaulus sp. with IE of 22.5%, Moniezia sp. with IE of 13.0%, Thysaniezia sp. with IE of 1.4%, Paramphistomidae with IE of 3.5%, and Eimeria sp. with IE of 28.3%. These values indicate a wide spread of parasitic nematodes of the gastrointestinal tract and lungs, which is consistent with the studies of Zhelyakova et al. (2016), Shalaeva (2017) and Loginova et al. (2018) in Russia. Thus, in comparison with wild and domestic reindeer of Taimyr, parasitic nematodes Nematodirella longissimespiculata (Shalaeva, 2017) and Ostertagia spp. (Zhelyakova et al., 2016) were not found in reindeer in the Khanty-Mansi Autonomous Region–Yugra. According to data of Loginova et al. (2018), in the adjoining territory of the Northern Urals (Yamalo-Nenets Autonomous Region), parasites Elaphostrongylus rangiferi and Parabronema skrjabini were discovered, which were not recorded in reindeer in the Khanty-Mansi Autonomous Region–Yugra, however, similar species were also found: Trichostrongylus sp. and Nematodirus spp. Imaginal cestodiases in reindeer caused by Moniezia spp., Avitellina arctica, and Thysaniezia giardi are common in Taimyr (Zhelyakova et al., 2016; Shalaeva, 2017) and the Yamalo-Nenets Autonomous Region (Siben et al., 2015). During our research, only Moniezia spp. was registered in the Khanty-Mansi Autonomous Region–Yugra, which may be due to different periods of the studies and the absence of complete helminthological autopsies of animals. Trematode infections, particularly of parasitic Paramphistomidae, were recorded in reindeer only in the Murmansk region (Pochepko, 2014) and in our study, which may be due to both the biological features of the pathogens and the wrong timing of the study for this kind of infection. Coccidia infection in reindeer is mentioned in the paper of Skírnisson and Cuyler (2015). It was also specified that only Eimeria rangiferis could infect this species. Based on that, we suggest that coccidian oocysts found in our study belonged to Eimeria rangiferis. Therefore, a one-time study of the reindeer parasite fauna in the Khanty-Mansi Autonomous Region–Yugra allowed us to develop knowledge on this matter. Based on this data, we recommend antiparasitic treatments for animals. However, such research is not enough and further extension of knowledge about invasive pathogens is required. It is necessary to pay special attention to parasites transmitted by blood-sucking insects, in connection with the change of their habitat boundaries (Domatsky et al., 2018).
CONCLUSION
It was observed that in the Khanty-Mansi Autonomous Region–Yugra, domestic reindeer are infected with nematodes of the gastro-intestinal tract and lungs (Trichostrongylus sp., Nematodirus sp., Trichocecpalus sp., Dictyocaulus sp.), imaginal cestodes (Thysaniezia sp., Moniezia sp.), trematodes (Paramphistomidae) and protozoa (Eimeria sp.). The average infection rate of animals in the region: Trichostrongylus sp.–31.8%, Trichocecpalus sp. –8.4%, Nematodirus sp. –12.4%, Dictyocaulus sp. –22.5%, Moniezia sp.–13.0%, Thysaniezia sp. –1.4%, Paramphistomidae–3.5%, Eimeria sp. –28.3%. Finally, use of pluripotential anthelminthic drugs, which are effective against nematodoses, cestodiases and distomiases of animals, is recommended in problem farms of the region.
ACKNOWLEDGMENTS
This article was prepared within the framework of the Fundamental Scientific Research Topic No. 0371-2018-0040 “Monitoring of the epizootic situation and forecasts of the development of possible outbreaks of parasitic animal diseases”.
ETHICAL PERMITS
Obtained from the ethical commission of All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology of the Tyumen Scientific Centre of the Siberian Branch of the Russian Academy of Sciences, comply with the national ethical requirements of the Russian Federation.
FUNDING SOURCE
Research was conducted with the financial support of the Russian Academy of Sciences within the framework of Fundamental Scientific Research Topic No. 0371-2018-0040 “Monitoring of the epizootic situation and forecasts of the development of possible outbreaks of parasitic animal diseases”.
CONFLICT OF INTEREST
There is no conflict of interest.
Authors Contribution
All authors contributed equally.
REFERENCES