Advances in Animal and Veterinary Sciences
Research Article
Experimental Extrahepatic Cholestasis in Dogs: Ultrasonographic, Biochemical and Histopathological Study
Mohamed Gomaa1*, Eman Metwally2, El Abas El Nagar3, Wafaa Abdel Razik3, Nora El Seddawy4, Yasmin Bayoumi3
1Surgery Department, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, El-Sharkia, Egypt; 2Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, El-Sharkia, Egypt; 3Animal Medicine Department, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, El-Sharkia, Egypt; 4Pathology Department, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, El-Sharkia, Egypt.
Abstract | To explore the role of ultrasound in the diagnosis of extrahepatic cholestasis in dogs, an experimental extrahepatic cholestasis was done successfully in seven male mongrel dogs via common bile duct ligation that mimic natural cases in dogs. All observations and changes were recorded post- operative; clinical, biochemical and ultrasonography. All dogs showed jaundice, orange colored urine and clay feces in the 3rd day post-operative. Significant increase in serum levels of AST, ALT, GGT, ALP, total and direct bilirubin post-operative started from the 3rd day till reaching the peak in the 2nd week, then declined again from the 3rd week post-surgery till reaching the lowest level at the 8th week. Meanwhile, there was a significant decrease in both total protein and albumin started from the 3rd day post-ligation and remained till the end of the study. Ultrasonography of biliary system showed significant dilatation of gallbladder and common bile duct starting from the 3rd day post-ligation till reaching the maximum in the 2nd week that showed a slight reduction in their diameter from the 3rd week and reached the lowest diameter in the 8th week post-ligation. Post mortem examination showed jaundice, emaciation, as well as gallbladder and common and cystic duct dilatation. Histopathology of liver showed subcapsular hemorrhage, congestion of central vein with vacuolation in hepatocytes and yellowish brown granules of bile pigment inside other hepatocytes. In conclusion, the usage of biochemical analysis and ultrasonography is very important in early diagnosis of extrahepatic cholestasis as the changes appeared within 3 days after obstruction.
Keywords | Mongrel dogs, Extrahepatic Cholestasis, Ultrasound
Received | September 11, 2019; Accepted | October 06, 2019; Published | October 10, 2019
*Correspondence | Mohamed Gomaa, Surgery Department, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, El-Sharkia, Egypt; Email: gomaasurgeon@yahoo.com
Citation | Gomaa M, Metwally E, El-Nagar EA, Abdel Razik W, El-Seddawy N, Bayoumi Y (2019). Experimental extrahepatic cholestasis in dogs: Ultrasonographic, biochemical and histopathological study. Adv. Anim. Vet. Sci. 7(s2): 44-50.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.44.50
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Gomaa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The main role of the extrahepatic biliary tract is mainly to collect, store, and concentrate bile, which formed by the liver and expelled to the duodenum via the common bile duct. The biliary system includes bile canaliculi, bile ductules, hepatic ducts, gallbladder, cystic duct, and common bile duct (Center, 2009). The majority of the secreted bile is diverted into the gallbladder where it stored which delivered to the duodenum through the cystic and common bile duct (Center, 2009). Cholestasis is a clinical and biochemical syndrome caused by an impaired bile flow mainly associated with clinical manifestations such as jaundice and itching, and biochemical disturbances such as elevated liver enzymes and serum bilirubin (Fernández et al., 2004). The mechanism of cholestasis is classified into hepatocellular, where an impairment of bile formation occurs, and obstructive, where the occlusion of bile flow occurs after its formation (Park et al., 2015). In case of hepatocellular cholestasis, the bile is lodged into the hepatocytes and canalicular spaces resulted in generalized cholate injury, while in obstructive cholestasis the bile flow is impaired and leading to distension of gallbladder and common bile duct (Park et al., 2015).
Abnormal accumulation of inspissated, semi-solid bile and/or mucous within the gallbladder lumen is called biliary sludge (Mesich et al., 2009; Norwich 2011). Gallbladder sludge is considered an incidental finding by radiologists, and can occur secondarily to stagnant biliary flow and gallbladder distension (Saunders et al., 2017).
When a common bile duct obstruction happened, the drainage of the bile into the duodenum impaired, and the bile accumulates in the ducts, bile canaliculi and hepatocytes which, consequently, dilate, induce the increase of liver size causing compression of hepatic cells in association with degenerative processes caused by cholestasis, and might progress to cellular death and cirrhosis (Cotran et al., 2005; Campos et al., 2013). The experimental induction of extrahepatic cholestasis leads to an obvious increase in the concentration of bilirubin in the blood circulation, deposition of such substance on tissues (jaundice) and glomerular excretion (bilirubinuria) (Cotran et al., 2005; Lassen, 2007).
Acute experimental cholestasis in dogs results in rapid elevation of serum alkaline phosphatase and bilirubin after 6 hours (Mehler and Bennett, 2006). Some studies identified the elevation of conjugated and total serum bilirubin levels 24 hours after experimental cholestasis and reported that total bilirubin levels reached their maximum at 5 to 6 days after ligation (Mehler and Bennett, 2006). However, serum liver enzymes elevation has no correlation with the degree of hepatobiliary injury or obstruction (Center, 1996).
Ultrasonography is very important diagnostic tool used to distinguish biliary obstruction from hepatocellular disease in clinically icteric animals (Crews et al., 2009). In experimental cholestasis in dogs, the ultrasonography revealed gallbladder distension, cystic duct was larger and more tortuous than normal, hypoechoic sludge was prominent in the gallbladder, and perivascular, multifocal hyperechoic areas appeared in the liver parenchyma (Gönül et al., 2002).
The purpose of this study is to explore the role of clinico-biochemical and ultrasonographic examinations in diagnosis of experimental cholestasis in dogs with the reference to pathological findings observed in the liver.
MATERIAL AND METHODS
Ethical statement and animals
This study was authorized by the Animal Experimentation Ethics Committee of Zagazig University (ZU– IACUC), under the number ZU–IACUC/2/F/59/2019.
This study was performed on seven mature male mongrel dogs aging from 6-11 months and weighing (20-25 kg). They were housed indoors in individual cages labeled with dog’s identifying; dogs were identified with an identification neckband. They were fed ad libitum on non-commercial diet (meat and chicken extract) with free access to food and water. Dogs were dewormed and vaccinated against viral diseases according to the regulation of veterinary authorities during the one-month acclimatization period. After that, all dogs were underwent the experimental study and common bile duct ligation was carried out surgically to induce extrahepatic obstructive cholestasis.
Experimental procedures
Preparation of animals: food withdrawal 8 hours before surgery but continue offering water until two hours prior to the operation. Dogs were sedated with xylazine HCL 2% (1 mg/ kg I/M), shaving and antisepsis on the epigastric region for the operation site. The animals were generally anesthetized using thiopental sodium intravenous (20 mg/kg bwt).
Surgical obstruction of common bile duct: The common bile duct was ligated at the duodenal region under general anesthesia. The surgical steps of common bile duct ligation were illustrated in Figure 1.
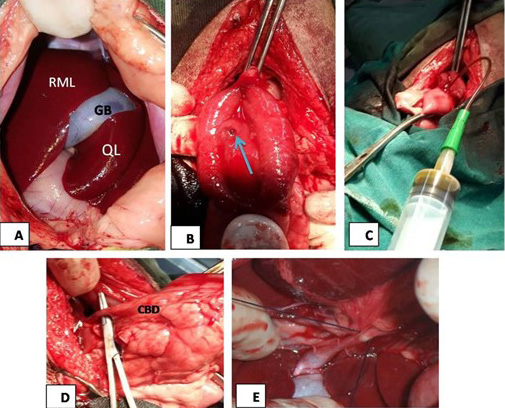
Figure 1: (A) The mid line incision at the epigastric region in liver area, RML: right medial lobe of liver, GB: gallbladder, QL: quadrate lobe; (B): image showed duodenal incision and exposure of major duodenal papilla (arrow); (C): the image showed bile aspiration; (D): the image showed common bile duct (CBD) exposure; (E): surgical ligation of common bile duct.
Postoperative care: After the surgery, dogs were housed in their separate kennels and daily observation to all animals was done. Follow-up and postoperative treatment with non-steroidal anti-inflammatory (Meloxicam ampules, dose; 0.2 mg/kg b wt, every 24 hours I/V) and antibiotics (cefotax® ampules, dose; 20 mg/kg bwt, every 12 hours I/V) was done to all dogs for 3 days, together with daily disinfection of the wound with Betadine 10%. Animals were kept on soft bedding with free access to food and water ad libitum.
Clinical examination and Biochemical analysis: Clinical examination to all dogs was performed according to Kelly (1984), special attention was paid to visible mucous membranes and sclera, closed observation to all cases during experimental periods. Blood samples were collected at zero day during the experimental period,3rd day, 1st, 2nd, 3rd, 5th and 8th week post-surgery. Five mL of blood was collected from each animal via cephalic vein puncture without anticoagulant into a clean dry centrifuge tube and clarified by centrifugation at 3000 rpm for 20 minutes to obtain clear sera. Serum AST, ALT, ALP and GGT levels were determined according to the methods described by Reitman and Frankel (1957), Bessy (1946), Tietz (1986), respectively. Serum Total and Direct bilirubin levels were estimated according to the method described by Malloy and Evelyn (1937). Serum total protein and albumin were estimated according to the method described by Doumas et al. (1971), Henary et al. (1974).
Ultrasonographic examination: Ultrasound examination was performed using a high definition ultrasound system equipped with 5–9 MHz linear transducer and 3-5 MHz micro convex Sonoscape A5V, China before surgery and during the experimental periods.
Post mortem and histopathological examination: Five dogs were euthanized at the end of the study and macroscopic alterations were recorded. Liver, gallbladder wall, common bile duct specimens were collected, washed with running water and fixed by immersion in 10% formalin solution for 48 hours. Paraffin sections 5-micron thickness where prepared and stained with H and E (Bancroft and Gamble, 2008) and examined microscopically using light microscope.
Statistical analysis
All data was evaluated on an individual basis by comparison with the base line data at zero day. Data were analyzed for statistical analysis using One way repeated measures ANOVA. All values were expressed as means ± standard error (SE). Statistical analysis conducted using SPSS 16.0 for windows (SPSS, Chicago, USA). The significance of the differences between average values was determined using Duncan’s test at significance levels P< 0.05 (Duncan, 1955).
RESULTS
Clinical findings
none of the animals suffered postoperative complications while all dogs showed jaundice, orange color urine and clay feces from the 3rd day post-surgery. Abdominal pain, excessive emaciation, weight loss, dehydration and reduced appetite were also recorded. Two dogs died within the 2nd week, one of them showed severe ascites and the other showed severe dehydration.
Biochemical analysis
The results of serum analysis during different experimental periods have shown the evidence of cholestasis within the first 3 days post-surgery (Table 1). Significant increase in the mean values of serum ALT, AST, GGT and ALP were recorded starting from the 3rd day till reached the peak at the 2nd week (p˂ 0.05), while their levels began to decrease from the 3rd week till reach the lowest levels at the 8th week compared to pre-surgical levels (Table 1). There were significant decrease in the mean values of serum total protein and serum albumin after common bile duct ligation starting from the 3rd day and extended till the end of the study (p˂ 0.05). There were significant increase in the mean values of serum total and direct bilirubin starting from the 3rd day till reaching the peak at the 2nd week post-surgery, then these levels showed gradual reduction from the 3rd week till reaching the lowest level at the 8th week (p˂ 0.05).
Ultrasonographic results
There was a significant distension of gallbladder started from the 3rd day post-surgery, which progressively increased in the 1st, 2nd and 3rd week showing a slight reduction in the 5th and 8th week in experimental dogs. A significant dilatation of the common bile duct diameter was also recorded in all cases from the 3rd day post-operative. that progressively increased in the 1st, 2nd and 3rd week, and then was progressively decrease in both the 5th and 8th week (Table 2 and Figure 2).

Figure 2: (A): Sagittal ultrasonographic image of gallbladder (GB) and common bile duct (CBD) in dog before surgery, 2.7mm; (B): the image showed the maximum dilatation of gallbladder (GB) and common bile duct in the 2nd week after ligation, 14.9mm.
Table 1: Mean ± standard error (SE) of serum biochemical parameters during different experimental periods.
| Experimental periods | ALT ( U/L) | AST (U/L) | GGT (U/L) | ALP ( U/L) | Total protein (g/dl) | Albumin (g/dl) | Globulin (g/dl) | Total bilirubin (mg/dl) | Direct bilirubin (mg/dl) |
| ZeroDay |
31.75±7.15c |
19.37±4.07e |
11.64± 2.20d |
119.37± 5.25c |
8.16± 0.40a |
3.72± 0.19a |
3.70± 0.45ab |
0.39±0.08c |
0.41±0.04d |
|
3rd Day |
55.98±11.26bc |
46.91±9.54cd |
58.77±5.08b |
969.53±9.29b |
6.89±0.18b |
3.01±0.70b |
4.61±0.24a |
3.14±0.49bc |
1.71±0.21c |
|
1st Week |
149.61±10.30ab |
62.08±10.16ab |
66.92±6.92ab |
972.94±4.89b |
6.64±0.19b |
2.98±0.29b |
3.66±0.30ab |
4.86±1.44ab |
2.82±0.41b |
|
2nd Week |
193.78±13.43a |
78.32±4.81a |
80.01±8.52a |
1809.01±10.74a |
6.14±0.33b |
2.31±0.15c |
3.89±0.38ab |
7.40±1.34a |
4.41±0.32a |
|
3rd Week |
144.09±8.47ab |
70.54±9.03ab |
40.58±2.96c |
721.60±8.22b |
6.48±0.03b |
2.68±0.07bc |
3.76±0.07ab |
5.01±1.13ab |
2.53±0.32b |
|
5th Week |
80.04±5.87bc |
50.08±5.98bc |
20.01±1.63d |
134.29±2.77c |
6.28±0.03b |
3.0±0.11b |
3.27±0.14b |
1.17±0.06b |
0.71±0.15d |
|
8th Week |
44.81±1.66c |
28.95±1.75de |
12.72±1.11d |
130.43±3.65c |
6.75±0.24b |
3.01±0.14b |
3.67±0.33ab |
0.87±0.04c |
0.61±0.14d |
Means in the same column with different small letters superscripts indicating significant difference at (p < 0.05) based on Duncan’s Multiple Range Test (DMRT) between different times in experimental group; Reference values ofALT (8.2-57 U/L); AST (15-66 U/L); GGT (1-12 U/L); ALP (5-131 U/L); Total protein (5.0-7.4 g/dl); Albumin (2.7-4.4 g/dl); Globulin (1.6-3.6 g/dl); Total bilirubin (0.1-0.4 mg/dl) and Direct bilirubin (0.1-0.4 mg/dl).
Table 2: Ultrasonographic gallbladder and common bile duct diameter from zero day till the end of the study.
| Time | Gallbladder diameter (mm) | Common bile duct diameter (mm) | |
| longitudinal axis | transverse axis | ||
| Zero day |
37.1±2.04c |
15.67 ± 0.56c |
2.9± 0.18c |
|
3rd day |
53.30± 1.80b |
31.10± 2.22a |
10.58 ± 1.20a |
|
1st week |
55.81 ± 4.21b |
32.90 ± 1.22a |
11.40 ± 1.09a |
|
2nd week |
64.14 ± 2.89a |
20.65 ± 1.98b |
12.75 ± 2.15a |
|
3rd week |
63.12 ± 4.01a |
25.76 ± 0.98ab |
11.2 ± 2.35a |
|
5th week |
60 ± 1.29ab |
22.4 ± 2.37b |
7.12 ± 1.45b |
|
8th week |
60.33 ± 3.12ab |
21.1± 3.25b |
6.44 ± 0.56b |
Means in the same column with different small letters superscripts indicating significant difference at (p < 0.05) based on Duncan’s Multiple Range Test (DMRT).
Post mortem and histopathological results
Severe emaciation, icteric mucous membrane, yellowish staining ribs, intestine and mesentery were recorded. Liver enlarged and slightly firm in consistency, enlarged gallbladder, common and hepatic bile ducts. Kidneys showed paleness color and softness in consistency. One dog showed ascites (intra-abdominal fluid effusion, about 10 liters), fibrinous peritonitis and adhesion in between abdominal viscera, and died in the 2nd week post-surgery.
Histopathological examination of the liver revealed subcapsular hemorrhage, congestion of central vein with vacuolation in some hepatocytes and yellowish brown granules of bile pigment inside other hepatocytes were also detected (Figure 3).
DISCUSSION
Experimental common bile duct ligation in this study resulted in cholestasis in dogs within 3 days post-surgery. Jaundice was the first sign observed in all dogs in visible mucous membranes and the skin which was attributed to increase the concentration of bilirubin in the blood owing
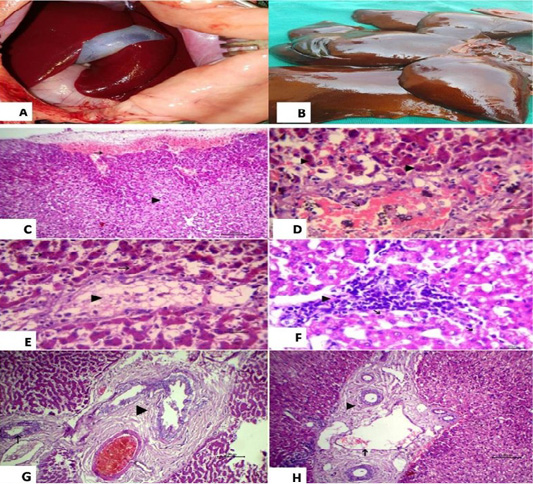
Figure 3: (A): showed normal liver before surgery; (B): Liver showed necrosis and firm in consistency after ligation and showed multiple grayish necrotic areas. Photomicrograph of HandE stained liver tissue; (C): subcapsular hemorrhage (arrow) and vacuolation in some hepatocytes (arrow head), 100 bar; (D): congestion of central vein (black arrow), vacuolation in some hepatocytes (white arrow) and yellowish brown granules of bile pigment inside other hepatocytes (arrow head), 20 bar; (E): fatty change of some hepatocytes (arrow head) and disassociation of other hepatocytes (white arrow) with yellowish brown granules of bile pigment inside hepatocytes HandE 20 bar; (F): the focal aggregation of lymphocytes with few macrophages infiltrations replaced necrotic hepatocytes (arrow head) and vacuolation in other hepatocytes, 20 bar; (G) Chronic cholecystitis represented by hyperplasia of its lining epithelium and thickening in the wall of gall bladder with fibrous tissue proliferation (arrow head) and chronic cholangitis (arrow), 100 bar; (H) Dilation of portal vein (black arrow) with chronic cholangitis represented by thickening in the wall of bile duct with fibrous tissue proliferation (arrow head), 100 bar.
to impairment of bile flow after common bile duct ligation and deposition of such substance on tissues mainly skin and mucous membranes. Prolonged obstruction of bile duct resulted in impairment of enterohepatic circulation and the feces become clay in color, leading to shifting the kidney to be the main route for its elimination that turns the urine to orange color, as previously described Wang and Wei-Feng (2014). Abdominal pain, excessive emaciation, diarrhea, reduced appetite and weight loss, all these symptoms could be attributed to impair bile flow in extrahepatic biliary tree that leads to retention of bile salts in liver and severe damage of hepatocytes, the animal showing different levels of hepatic dysfunction, these findings agreed with Rothuizen and Meyer (2000), Bunch (2003), Otte et al. (2017).
Ascites was observed during this study in one case, this abdominal effusion resulted from excessive accumulation of unconjugated bile acids in blood. Unconjugated bile acids are cytotoxic and induce tissue inflammation and alter the permeability of vascular structures within the peritoneum leading to transudation of fluid into the peritoneal cavity, these results previously mentioned by Owens et al. (2003) and we can assume that the two cases that died in the 2nd week post ligation were suffered from hepatorenal syndrome type 1 (HRS) that is highly fatal.
Regarding the biochemical alterations in this study, a highly significant increase in the levels of liver enzymes in different experimental periods started from the 3rd day. ALT and AST are hepatocellular enzymes found mainly in the hepatocytes and released into circulation in case of hepatocellular membrane damage. The main reason for the obvious increase in liver enzymes is the severe damage occurred to the liver tissue secondary to common bile duct ligation and their leakage into blood stream, these results were similar to the results recorded by Jiang et al. (2016), who mentioned that liver enzymes were significantly increase 3 days after common bile duct ligation. Also, the serum GGT and ALP showed significant increase after common bile duct ligation, this increase is attributed to marked damage occurred in biliary system secondary to severe distension of cystic, hepatic and common bile ducts due to extensive accumulation of bile and prominent leakage of these enzymes to systemic circulation, these findings agreed with Setyawan and Budipramana (2015), who mentioned that the increased levels of GGT and ALP occurred three days after ligation of the CBD, then fluctuated until day 29.
Significant decrease in the mean values of Serum total protein after ligation in experimental group started from the 3rd day, then the level showed a slight increase again at the 3rd week compared to 0 day level, these findings agreed with Mwanza et al. (1998), who recorded that common bile duct in dogs resulted in a steady decrease in serum total protein throughout the examination period that lasts for 10 weeks. Hypoproteinemia observed in this study mainly due to severe damage of hepatocytes secondary to biliary stasis because the liver is the main site for synthesis and degradation of the proteins in the body.
Albumin is a plasma protein mainly synthesized by the liver and considered an important marker of hepatocellular function. This detectable change may reflect a reduction in hepatic synthesis associated with bile duct obstruction. So this significant reduction in albumin level usually indicates liver damage secondary to extrahepatic cholestasis, these findings were in accordance with Mohamed et al. (2015).
Significant increase in the mean values of serum total and direct bilirubin after ligation starting from the 3rd day till reach the peak at the 2nd week post-surgery, then the level began to decrease from the 3rd week till reaching the lowest level at the 8th week compared to 0 day level, these results were in accordance with that obtained by Crema et al. (2007), who recorded that common bile duct ligation in dog resulted in increase in total bilirubin after one week post-surgery. Setyawan and Budipramana (2015) mentioned that the level of direct and total bilirubin showed significant increases from the 3rd day, with a reduction on day 14, and increased again until day 29.
Ultrasonographic findings revealed a significant distension of gallbladder and biliary tree started from the 3rd day post-surgery and this distension progressively increased after there. The surgical ligation of common bile duct leads to occlusion of the normal pathway of bile in the extrahepatic biliary system and then the bile returns again to the gallbladder and intrahepatic ducts. This change in bile pathway results in significant distension of gallbladder and obvious dilatation of cystic, hepatic and common bile ducts, which can be easily detected during the ultrasonographic examination. These results were previously mentioned by Vergine et al. (2005), as he reported that ultrasonography of dog suffered from common bile duct obstruction showed a diffuse increase in echogenicity of the liver, with severe gallbladder distension and marked dilation of the cystic duct, common bile duct and extrahepatic bile ducts.
Histopathological observations in experimental group revealed that the normal histological architecture of liver was completely lost after surgical ligation of common bile duct and these changes were attributed to bile retention in hepatocytes and subsequent damage to liver tissue after biliary stasis; which is was similar to previous findings recorded by Awad et al. (2000), Aller et al. (2008), Panqueva (2014).
CONCLUSION
Induction of extrahepatic cholestasis via surgical ligation of common bile duct in dogs is considered the most reliable model that mimic the natural cases of extrahepatic cholestasis. The use of ultrasound in extrahepatic cholestasis is very relevant, helping in the early identification and evaluation of the obstruction, and the usage of biochemical analysis is very important in early diagnosis as the changes appeared within 3 days after obstruction.
ACKNOWLEDGEMENT
This work was supported by Faculty of Veterinary Medicine, Zagazig University, Egypt.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHORS CONTRIBUTION
All authors contributed equally in this work.
REFERENCES





