Advances in Animal and Veterinary Sciences
Research Article
Residues of Anticoccidial Drug (Diclazuril) in Different Broiler Tissues by High Performance Liquid Chromatography
Ahmed Abdou Said1, Sameh M. El-Nabtity1, Adel M. Abd El-Aziz2, Eman I. Elassal2*
1Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt; 2Animal Health Research Institute, Tanta, Egypt.
Abstract | The controlled usage of veterinary drugs is a necessity not only for the wellbeing of the animals but also for the quality and safety of human food. The diclazuril is among the commonly administered anticoccidials in veterinary medicine for combating avian coccidiosis, which may lead to its accumulation in poultry’s tissues and consequently may possess potential hazards on human consumer’s health. Accordingly, this trial was designed to detect residues of diclazuril in different broiler tissues and to define its withdrawal period to ensure the safety for human consumption. A total of 150 broiler chicks of one day old were grouped into several groups; Group II: was orally gavage diclazuril at a dose rate of 0.3 mg/kg bodyweight once daily for 3 successive days only at age of 15th day old, group III: was orally gavage the same dose once daily for 3 successive days at age of 15th day old and for another 3 successive days at age of 32nd, finally group IV: was orally gavage the same dose once daily for 3 successive days only at age of 32nd day old. One day post last diclazuril administration and for 7 successive days, five chicks were slaughtered daily from all treated groups and samples from muscle, liver and kidney were obtained to be analyzed by high performance liquid chromatography (HPLC). Diclazuril was detected in muscles, kidney and liver of all treated groups. Whereas the detected concentrations were below the permissible limits, so it was concluded that there is no premarketing withdrawal requirement for diclazuril. Also neither the age of the bird, nor the repeated doses of diclazuril with time interval affected the accumulation of the diclazuril in broiler tissues. Further studies needed to monitor the effect of longer administration period of diclazuril on its accumulation in different broiler tissues.
Keywords | Residues, Diclazuril, Broiler, High performance Liquid chromatography
Received | September 11, 2019; Accepted | October 06, 2019; Published | October 10, 2019
*Correspondence | Eman I. Elassal, Animal Health Research Institute, Tanta, Egypt; Email: eman.elassal89@gmail.com
Citation | Said AA, El-Nabtity SM, El-Aziz AMA, Elassal EI (2019). Residues of anticoccidial drug (diclazuril) in different broiler tissues by high performance liquid chromatography. Adv. Anim. Vet. Sci. 7(s2): 19-25.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.19.25
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Said et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The continuous spreading out of livestock industry which is tightly bound to universal food production compel the control of coccidiosis which is considered the most ungovernable prevalent disease of poultry caused by coccidian protozoa Eimeria of the phylum Apicomplexa, (Noack et al., 2019). Coccidiosis causes extensive damage to the intestinal lining of the bird, and while many infections are subclinical, the clinical signs typically include diarrhea, decreased growth rate, decreased feed and water intake, decreased egg production, and increased mortality (Gerhold, 2019).
The main method of controlling coccidiosis, in conjunction with strict hygiene and biosecurity methods, is through the addition of small, precisely measured amounts of anticoccidials in the feed (Kadykalo et al., 2017). However, the intensive use of these drugs either when they are administered orally or mixed with feed and water usually give rise to the accumulation of their residues in food products intended for human consumption (Basha et al., 2016). In consequence of the widespread emergence of antimicrobial resistance, concerns have been raised regarding the safety of anticoccidials and potential impact on human health (Kadykalo et al., 2017). Literature survey revealed that diclazuril (4 -Chlorophenyl) [2, 6-dichloro -4- (3, 5-dioxo-4, 5-dihydro-1, 2, 4-triazin-2(3H)-yl) phenyl] acetonitrile) is official in British Pharmacopeia (2013).
Diclazuril is one of triazine groups, which comprise benzene-aceto-nitrile anticoccidials agents widely used to combat the detrimental effects of coccidosis (Stock et al., 2017). Although the exact mechanism of action is currently not fully understood, diclazuril is suggested to exert its beneficial effects as a coccidiocidal compound through targeting the sexual and asexual stages of Eimeria (Verheyen et al., 1989; Zhou et al., 2010; Wang et al., 2013). However, the irrational or intensive usage of diclazuril may result in residues in animal products, consequently not only cause potential hazards to human health but also increase drug resistance of coccidian (Zhang et al., 2019 a).
Whereas the acute toxicity has never been observed in human, there is a great concern directed toward the chronic toxicity arising from the long-term exposure to low levels, so Codex Alimentarius Commission (CAC, 2018) has established maximum residue limits for diclazuril in poultry to be 500 ppb for muscle, 2000 ppb for kidney and 3000 ppb for liver. Due to the global use of anticoccidial agents, the development and validation of useful methods for determination of these compounds is a concern for food and analytical chemists (Barreto et al., 2017). Liquid-chromatography (LC) has proven to be an optimal technology for screening, detection and quantification of a vast variety of analyses where within the LC approach itself several alternatives are available (Cortés-Herrera et al., 2018).
The importance of detection of diclazuril in different poultry edible tissues using high performance liquid chromatography (HPLC) was evoked due to the enduring or irrational off- label use of diclazuril in poultry farmers for combating coccidiosis and so its potential accumulation in different edible tissue above permissible limits; so this trial designed to determine the withdrawal period of diclazuril which is established to ensure that is no longer drug residue at slaughter time hence can avoid potential hazards to human consumers.
MATERIALS AND METHOD
Chemicals and standard solutions
Chemicals: Diclazuril analytical grade with purity ≥ 98% and methanol for HPLC ≥ 99.9 % were purchased from Sigma -Aldrich Co. (Steinheim, Germany); HPLC grade acetonitrile (ARLO ERBA reagent, Spain).
Stock and working solutions of diclazuril: Standard stock solutions of diclazuril were prepared by weighing approximately 5 mg within a glass tube and adding an appropriate amount of dimethylformamide as solvent to reach a concentration of 1 mg/ mL. The stock solutions were stored at 4 ºC.
Working standard solutions: Working standard solutions of 50, 100, 200, 500, 1000, 2000 and 5000 ng/mL of diclazuril were prepared by diluting the stock solutions in distilled water for preparation of calibration curve and spiking.
Drug
Diclazuril: Liquid diclazuril 0.5% (Luxi) manufactured by Shandong luxi animal medicine share company, Ltd.
Experimental birds and study protocol: The study obtained ethics approval from Zagazig University Institutional Animal Care and Use Committee with approval ID “ZU-IACUC/2/F/118/2019”. A total of one hundred fifty apparently healthy one day-old broiler chicks (Indian River) were reared on cleaned and disinfected wire floor cages. Feed and water were provided ad libitum without any feed additives or anticoccidial agents.
Chicks were divided randomly into one control group (I) contained 15 chicks which kept without any treatments for preparation of spiked samples and another equal 3 treated groups each of 45 chicks. The chicks were reared until age of 45th day old.
All treated groups were orally administered the recommended therapeutic dose of diclazuril (0.3 mg/kg b.wt) (EPMAR, 2013) once daily for 3 successive days. Chickens were gavage orally directly into the crop using a flexible plastic tube fitted to 5 mL syringe.
Group II: on the 15th day old, chicks were orally gavage the recommended therapeutic dose of diclazuril.
Group III: on the 15th day old, chicks were orally gavage the recommended therapeutic dose of diclazuril, and then later on the 32th day old chickens were re- gavage diclazuril with the same used regime.
Group VI: on the 32th day old, chicks were orally gavage the recommended therapeutic dose of diclazuril. In all treated groups, one-day post treatment and for 7 days, five chickens from each group were randomly selected; slaughtered and edible tissues (muscles, liver and kidneys) were obtained. The samples were kept in foil and frozen at -20 ºC until the time of qualitative and quantitative analysis of diclazuril using HPLC. The same was done for the blank samples obtained from the control group.
Preparation of samples
Preparation of samples from treated groups for diclazuril extraction from edible tissues: Diclazuril was extracted from different poultry tissue according to the method described by Mortier et al. (2005) where within a centrifuge tube, 2 grams of minced tissue was weighed and then 6 grams of anhydrous sodium sulfate was added and mixed with the tissue. Ten mL of acetonitrile was added and to homogenize the material and solvent; the tube was vortex-mixed. Shaking for 30 min was done followed by centrifugation at 4000×g for 15 min. Five milliliters of supernatant was transferred into a tube and under nitrogen in a water bath at 60 ºC it was evaporated to dryness. Using 1 mL a mixture (50/50, v/v) of (acetonitrile/water) containing 0.1% formic acid; the sample was re-dissolved. Filtration through a 0.22 µm filter was done, and then 10 µL from the extract was injected into the liquid chromatography system to be analyzed by LC/UV system.
Preparation of spiked samples for method validation: In order to prepare spiked samples, different standard solutions concentrations of diclazuril (50, 100, 200, 500, 1000, 2000 and 5000 ng/mL) were injected into a homogenized blank meat, liver and kidney obtained from chickens of control group and then treated according to the proposed method.
Chromatography
HPLC system: The chromatographic conditions and mobile phase consistence were done according to Kanda et al., (2003) where the liquid chromatography separation was performed using an Agilent series 1200 (Hewlett- Packard, Les Ulis, France), equipped with a (C 18, (250mm x 4.6mm ×5.0μm)) column, and acetonitrile; ammonium acetate containing 0.01 mol/L tetra-n-butylammonium hydrogen sulfate (43: 57) as the mobile phase. Flow rate: 1mL/min; column temp.: 40°c; detection: UV 280; injection volume: 10 µL.
Preparation of calibration curves
Working standard solutions of 50, 100, 200, 500, 1000, 2000 and 5000 ng/mL of diclazuril were prepared as mentioned before then the calibration curves were obtained through plotting the different concentrations of diclazuril versus its corresponding peak areas (obtained by HPLC).
Method of Validation: It is a process established by laboratory studies to ensure that the performance characteristics of the method meet the requirements for the intended analytical application according the International Conference on Harmonization (ICH, 2005).
Linearity and range: The linearity in chromatography within the working range of diclazuril (50, 100, 200, 500, 1000, 2000 and 5000 ng/mL) was scaled by calculation of regression coefficient value (R2) using the calibration data.
Limit of detection (LOD) and limit of quantification (LOQ): The detection limit and the quantification limit for diclazuril were calculated based on standard deviation (SD) of response and the slop. LOD and LOQ = Standard deviation of y-intercepts of the regression line / slop of calibration line) multiplied by 3.3 and 10 respectively.
Method precision (repeatability and intermediate precision): The test concentration 1000 ng/mg of diclazuril was assayed for 6 times (n=6) within the same day and 3 times (n=3) for 3 sequent days to appraise the intraday and inter-day precision respectively. The obtained values represented as relative standard deviation %.
Specificity: Specificity is the ability of a method to discriminate between the analyst of interest and other components that may present in the sample .The specificity of the method was evaluated via testing peaks purities of diclazuril where a blank muscle sample, diluted pure standard of diclazuril (500 ng/mL) and spiked muscle sample with the same concentration were injected to the chromatography system to observe if there are any interfering peaks at the same retention time.
Accuracy (recovery): The more nearness between the accepted true value and the actual result obtained from the spiked tissues analysis, the more accuracy of the intended method. Blank broiler tissues spiked at 3concentration levels (½ MRL, 1 MRL and 2 MRL) were used to calculate the recovery rates of diclazuril to evaluate the accuracy of the intended method. The spiked samples were treated according to the same described extraction procedure for diclazuril, injected to the chromatography system and the recoveries values % was calculated.
Robustness: The notion of the intended method to be sturdy or robust is coming from the ability of the method to withstand slight variation in the used system condition. It was assured by changing in some instrumental characteristics like the wavelength, column temperature and the flow rate. The results represented as relative standard deviation percentage.
System suitability test: To test the suitability of the used system to detect diclazuril using the intended method six injections of standard solution (1000 ng/mL) of diclazuril was done. According to the interpretation of the relative standard deviations of retention time, tailing factor, number of theoretical plates, peak area, and capacity factor measurements the system suitability is defined.
Statistical analysis: Data were presented as means plus or minus the standard error (Mean±SE). The data were analyzed statistically by one-way ANOVA test according to statistical analysis System User Guide SAS (2004) software and probability of (p<0.05) to compare between different diclazuril treated groups at different time.
RESULTS
Optimization of used chromatography conditions
The best retention time and good resolution of diclazuril peaks gained through experiments varying in some conditions like the mobile phase and the flow rate by using of the standard solutions of diclazuril. The best resolution was found using a mixture of acetonitrile; ammonium acetate (43:57) as a mobile phase. The most efficient flow rate 1 mL/min with an injection volume of 10 µL, the column temperature was maintained at 40°c and UV detection wavelength was set at 280 nm.
Calibration curves
As declared in Figure 1, the calibration curve was linear within the tested range of diclazuril (50 -5000 ng/mL).
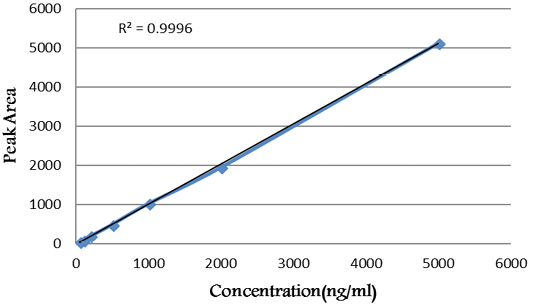
Figure 1: Calibration curve of diclazuril.
Method validation
In conformity of the ICH guidelines, the proposed method showed its outstanding validity for detection of diclazuril.
Linearity: The linearity regression equation was (y = 1.023 * × + 11.166) in the concentration ranges of 50-5000 mg/µL of diclazuril. The linearity of calibration curves (Figure 2) declared that the method showed good linearity where the value of regression coefficient (R2) was 0.9998.
LOD and LOQ: Based on calibration curves and the regression equation, the (y) intercepts of the regression line and the slope of the calibration curve were 11.166 and 1.023 respectively. Hence, the calculated value of the detection limit and quantification limit of diclazuril were 5.18 ng/mL and 15.71 ng/mL respectively.
Specificity: The representative chromatograms of the blank muscle sample, standard solution and spiked sample in Figure 2 (a, b, c) asserted that the method was specific for diclazuril detection where there were no intruding peaks at the same retention time of diclazuril except those for diclazuril. The retention time (R.T.) of diclazuril was 7.88 minutes.
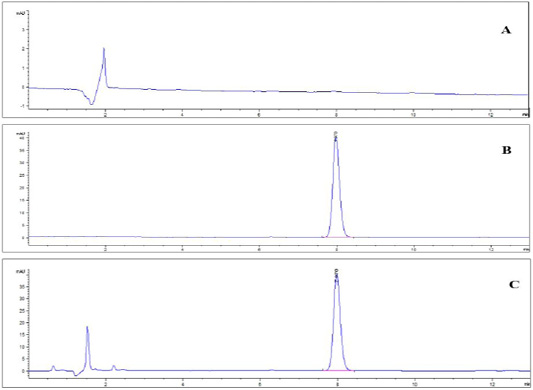
Figure 2: HPLChromatogram of (A) blank muscle sample; (B) diclazuril pure standard solution at a concentration of 500 ng/ml. and (C) spiked muscle sample at a concentration of 500 ng/gm.
Accuracy: The average rates of recovery % of diclazuril strengthened at 3 different concentrations levels were in the range of (98.93 % - 100.05 %) in different tissues. The obtained values of the relative standard deviation% hit the accepted value settled by ICH (≤2.0%) which assured acceptable accuracy of the method for detection of diclazuril.
Method precision: The obtained RSD% values for intraday and inter-day precision studies for diclazuril were 0.73 % and 0.54 %, respectively. These values were lower than those required by ICH guidelines (< 2 % and < 5 %) for intraday and inter-day analyses. Hence, the precision of the developed method was showed to be true.
Robustness: Whereas the detected amount of diclazuril not affected by the variation in all parameters intended for the method and the pooled RSD% value of the tested parameters was (<2%) which come in contact with ICH guidelines, it is a strong evidence on the ability of intended method for diclazuril detection to robust the changes in different operating parameters.
System suitability test: ICH guidelines recommended value for relative standard deviations % of the tested parameters in system suitability test is < 2. Table 1 declared the relative standard deviations % of the tested parameters which were as required by ICH. This confirmed the outstanding suitability of the used system for diclazuril detection.
Table 1: Results of system suitability test of diclazuril.
| Parameters | ||||
| Injection | Retention time | Tailing factor | Theoretical plates | Peak area |
| 1 | 8.021 | 1.01 | 9978 | 1029.7 |
| 2 | 7.883 | 1.001 | 10217 | 1019.9 |
| 3 | 7.987 | 0.998 | 9977 | 1035.3 |
| 4 | 7.98 | 0.999 | 10000 | 1022.8 |
| 5 | 8.01 | 1 | 10100 | 1023.02 |
| 6 | 7.789 | 0.983 | 9999 | 1027.29 |
| Mean (n = 6) | 7.945 | 0.99 | 10045.166 | 1026 |
| STDEV | 0.090741391 | 0.008734987 | 95.77351756 | 5.61250657 |
| RSD (%) | 1.14 | 0.87 | 0.95 | 0.54 |
Residues of dicalzuril in different broiler tissues
Diclazuril was detected in the muscles of all treated groups till the 3rd day post dosing but there was no significant difference between the three treated groups (P< 0.05). The residues (ppb) depleted from 787.67 in the 1st day to 393.81 in the 3rd day, from 770.33 in the 1st day to 416.83 in the 3rd day and from 788.04 in the 1st day to 434.18 in the 3rd day for group II, III and IV respectively. While the residues extended in liver and kidney tissues till the 4th day post dosing of diclazuril. In liver the residues (ppb) depleted from 3850 in the 1st day to 595.26 in the 4th day, from 4193.50 to 571.93 and from 4238.47 to 519.31 for group II, III and IV respectively. In kidneys the residues (ppb) depleted from 3063.33 in the 1st day to 464.17 in the 4th day, from 2879.53 in the 1st day to 451.66 in the 4th day and from 2908.33 in the 1st day to 410.11 in the 4th day for group II, III and IV respectively. Also muscles contained the lowest concentration of residues while the liver contained the highest. Means of diclazuril residues in different tissues were outlined in Table 2.
DISCUSSION
The controlled usage of veterinary drugs is a necessity not only for the wellbeing of the animals, but also for the quality together with the safety of food intended for human consumption (Hagren et al., 2005).
Pervious literatures were brimful with the preventive and therapeutic effects of diclazuril; as an effective triazine anticoccidial drug combating Eimeria infections in chickens (El-Banna et al., 2005; Zhang et al., 2019 b).
Prophylaxis is always the preferable plan to control coccidiosis in poultry as once the clinical signs become apparent, the treatments will be too useless to prevent the pathological sequels of the infection (Chapman, 2009).
The concept of prevention of coccidial infection in poultry was done in this trial by oral administration of diclazuril at different ages to apparently healthy chicks.
The results emphasized that neither the age of the bird nor the repeated doses of diclazuril with time interval affected the accumulation of the diclazuril in different poultry tissues as there was no significant difference between treated groups at different days. This may be attributed to the pharmacokinetic proprieties of diclazuril; whereas although dicalzuril characterized by extensive distribution, it is rapidly eliminated from chicken tissue after oral administration (Zhang et al., 2019a) this is suggested that diclazuril not a cumulative drug.
Residues of diclazuril were detected in the muscles of all treated groups till the 3rd day post dosing. While the residues extended in liver and kidney tissues till the 4th day post dosing of diclazuril. Also muscles contained the lowest concentration of residues while the liver contained the highest. Pharmacokinetic data show that for broilers, the liver is the target tissue, that is, the highest residue concentrations are found in the liver (Scan, 1997).
These results were in accordance with those reported in EMEA (1996) where administration of diclazuril orally to broilers at a dose rate of 90 μg/ kg daily for 14 days cause its accumulation within muscle and liver at a concentration of 40 and 240 ppb respectively after 24 of last dosing and depleted to reach 20 and 200 ppb in muscle and liver respectively after 72 hr of last dosing.
In the same line a tissue residue depletion study reported in EPMAR (2013) where the birds were given oral doses of 0.3 mg diclazuril/kg bw/day for 3 consecutive days in feed; the residues of diclazuril were the highest within the liver followed by the kidney. The mean residue levels in broilers depleted from 1443 ppb in liver, 1208 ppb in kidney and 209 ppb in muscle after last administration (0 hour) to 565 ppb in liver, 446 ppb in kidney, and 101 ppb in muscle after 48 hours of last dose.
While these results was not entirely consistent with the previous study of Zhang et al., (2019a) where although the highest diclazuril levels were detected in kidney and liver and the lowest were detected in the muscles, diclazuril not detected in the muscles on the 6th day post dosing and on the 8th day in liver and kidney following the withdrawal of drinking water medicated with 5 mg/L racemic diclazuril. This may be attributed to the longer period of drug administration 7 days via drinking water which may lead to more residual time or due to different LOD (8 ng/g) and LOQ (25 ng/g) by the chromatography system.
Table 2: Means of diclazuril concentration in muscle, liver and kidney of broilers (ppb).
| Muscle | Liver | Kidney | |||||||
| Groups | Group II | Group III | Group IV | GroupII | GroupIII | GroupIV | GroupII | GroupIII | GroupIV |
|
1st |
787.67 ±42.85 |
770.33 ±41.90 |
778.04 ±42.33 |
3850 ±210 |
4193.50 ±230.88 |
4238.47 ±231.19 |
3063.33 ±158.21 |
2879.53 ±148.72 |
2908.33 ±150.21 |
|
2nd |
606.50 ±32.99 |
600.43 ±32.67 |
552.40 ±30.05 |
3076.15 ±167.79 |
3045.39 ±166.11 |
2801.76 ±152.82 |
2450.66 ±126.57 |
2426.16 ±125.30 |
2232.06 ±115.28 |
|
3rd |
393.81 ±33.27 |
416.83 ±39.69 |
434.18 ±36.68 |
1828.40 ±249.74 |
1919.82 ±262.23 |
2015.81 ±275.34 |
1228.24 ±159.56 |
1191.39 ±154.78 |
1155.65 ±150.13 |
|
4th |
dedected | dedected | dedected |
595.26 ±87.82 |
571.93 ±86.94 |
519.31 ±78.95 |
464.17 ±73.77 |
451.66 ±73.12 |
410.11 ±66.39 |
|
5th-6th |
dedected | dedected | dedected | dedected | dedected | dedected | dedected | dedected | dedected |
Values are represented as mean ± SD; No significant difference between treated groups (P< 0.05).
To control the presence of unwanted drug residues and guarantee the food safety, the food safety authorities have settled maximum residue limits for varieties of veterinary drugs, also defined withdrawal periods at which the levels of residues in the tissues fall below the stated MRLs to guarantee complete the protection of consumers from potential hazards may arise from food (Hagren et al., 2005).
As showed in Table 2, the detected values of diclazuril were below the permissible limit established by the Codex Alimentarius Commission, CAC (2018). Consequently, no premarketing withdrawal period is required for diclazuril when administered at a dose rate of 0.3 mg/kg b.wt for 3 days.
CONCLUSION
The proposed method showed its outstanding validity for the detection of diclazuril in various broiler tissues and can be used to determine under the MRLs levels. Oral administration of the therapeutic dose of diclazuril 0.3 mg/kg body weight for 3 days causes accumulation of diclazuril residues in muscle tissue of broiler for 3 days post administration while extend to 4 days in liver and kidney but still below permissible limits. As a consequence, there is no premarketing withdrawal requirement for diclazuril. Neither the age of the bird, nor the repeated doses of diclazuril with time interval affected the accumulation of the diclazuril in different broiler tissues.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
Authors contribution
Ahmed Abdou Said and Sameh Mohamed El-Nabitiy: Study conception and design.
Adel Mohamed Abd Alaziz and Eman Elassal: Acquisition of data.
Sameh El-nabtity and Eman Elassal: Analysis and interpretation of data.
Dr. Eman Elassal: Drafting of manuscript.
Sameh Elnabtity and Adel Abd Elaziz: Critical revision.
REFERENCES





