Advances in Animal and Veterinary Sciences
Research Article
Detection of Parasites of Animal Health Importance Carried by Wild Rats Collected from Jeddah City, Saudi Arabia
Ahmad Allam1*, Eid Saed Ibrahim2, Adama Kazienga3, Fahad Al Zahrani4
1National Research Centre, Egypt; 2Parasitology Specialist, Laboratory of Parasitology. Public Health Pests Laboratory Project, Jeddah, KSA, Saudi Arabia; 3Statistics Specialist, Public Health Pests Laboratory Project, Jeddah, KSA, Burkina Faso; 4Home Pest Control Manager. Municipality of Jeddah, KSA, Saudi Arabia.
Abstract | Surveillance was conducted on the wild rats in Jeddah city, Saudi Arabia to explore the circulating parasites of animal health importance carried by it. A total 839 of three species of wild rodents, Brown rat (n. 742), Black rat (n. 94) and House mouse (n. 3) were live-captured. All received rats were euthanized and grossly examined. Dead rodents were carefully brushed to collect ectoparasites. The viscera were examined macroscopically and microscopically for any evidence of worms. The livers were examined for the presence of cysts. Concentration procedures were applied to separate parasites and its eggs from fecal debris. Blood on EDTA from the heart was stained with Giemsa and examined for the presence of blood flagellates. The Rattus norvegicus was the most predominant with 742 (88.44%) and followed by the Rattus rattus and Mus musculus with 94 (11.20%) and 3 (0.36%) respectively. The ectoparasites identified during the study were fleas 114 (13.59%), mites 43 (5.13%) and ticks 7 (0.83%). A total of 174 (20.74%) was assessed with positive of endoparasites. The Cysticercus fasciolaris, the larval form of Taenia taeniaeformis, cestode parasite of cats, was the predominant endoparasites identified with 115 (13.7%). Hymenolepis nana & Hymenolepis diminuta were found in 68 (8.1%) and 9 (1.07%) respectively in live-captured rodents. The overall infestation with Trypanosoma was 18.6%. There was a very high significance in Trypanosomiasis between males and females rats (p > 0.001).
Keywords | Endoparasites, Ectoparasites, Rattus spp., Trypanosoma, KSA
Received | June 23, 2019; Accepted | August 10, 2019; Published | September 25, 2019
*Correspondence | Ahmad Allam, National Research Centre, Egypt; Email: ahmdallam@gmail.com
Citation | Allam A, Ibrahim ES, Kazienga A, Al Zahrani F (2019). Detection of parasites of animal health importance carried by wild rats collected from jeddah city, Saudi Arabia. Adv. Anim. Vet. Sci. 7(10): 921-928.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.10.921.928
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Allam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Wild rodents spread a large number of diseases and they live in close contact with animals and humans. It can transmit disease through its excreta or insects on it (Bonnefoy et al., 2008). It can intensify the pathogens and get them into direct contact with animals and humans. In addition, they can carry many different protozoa and helminths (Claveria et al., 2005).
Rodent-borne diseases can be dispersed via direct routes such as biting and consumption of contaminated food and water. Moreover, wild rodents can retain pathogen transmission cycles in different conditions, varying from densely populated areas to rural areas and wilderness as well (Meerburg et al., 2009). Many endo and ectoparasites use wild rodents as intermediate and/or definitive hosts. Several species of these parasites are common to domesticated animals (Singla et al., 2008). These pathogens include bacteria such as Leptospira, Yersinia and Rickettsia spp. (Costa et al., 2014). Besides, viruses such as rat rotavirus B & A strain where its sequences showed close identity to animal strains (Sachsenröder et al., 2014).
Endoparasites were also detected such as cestodes (Fagir and El-Rayah , 2009). Hussein et al. (1991) recorded a circulation of different species of Trypanosoma in indigenous farm animals in different localities in Saudi Arabia. Trypanosoma lewisi was observed in Rattus norvegicus trapped in Egypt (Abdel-Aal and Abou-Eisha, 1997) and in Brazil (Linardi and Botelho, 2002). Fewer records of Trypanosoma evanci in rodents were recorded. The close association of rodents with livestock and their exposure to bloodsucking arthropods amplifies the opportunity of transmission of parasites. Efforts in mapping the geographical distribution of rats-related disease epidemiology are required to elucidate the existing and/or future threats in Saudi Arabia.
This work aimed to run comprehensive surveillance to disclose the circulating parasites of animal health importance carried by species of rats found in Jeddah city, Saudi Arabia. It will aid build prevailing control programmes that are effective and has a return on both animals and humans in terms of one health concept.
Materials and Methods
Rodents’ Collection
A total 839 of three species of wild rodents, Brown rat (n. 742), Black rat (n. 94) and House mouse (n. 3) were live-captured from wet markets, drains behind restaurants, food courts, houses and public areas by a single and multi-catch traps. The study was run on rats collected between December 2015 and June 2017.
Plans and strategy for traps distribution in all 14 municipalities of Jeddah city were established by the Management and Control Company through trained staff and specialists. Traps were placed along the walls, on rodent runways, near rodent burrows and other activity sites. Rats were trapped at night and conveyed to the laboratory in the next morning.
Euthanasia
All received rats were euthanized where each trap containing the live rat inside was individually placed into a big bucket with a lid. The animals were euthanized by carbon dioxide gas asphyxiation with extra confirmation of death by head dislocation. (AVMA guidelines for the euthanasia of animals: 2013 edition).
Gross Examination
Each euthanized rat was grossly examined for unusual skin wounds, gender, weight (approximate) and length (with/without tail) of each rat was recorded. Data locality of each rodent was recorded separately as received from the control team. Then, euthanized rodents were carefully brushed on a white plate and the collected ectoparasites were taken by a moistened hairbrush and kept in 70% ethanol in separate vials. Some of the brushed hair was transported to the lab for further microscopic examination.
Necropsy
The euthanized rats were incised in midventral line to expose and examine the viscera macroscopically. The gastrointestinal tract was exposed and small/large intestine is examined for the presence of worms. The cecum and colon were placed in a petri dish with normal saline to keep it wet. These viscera were opened under a dissecting microscope and examined by using saline to ease worm identification. The intestinal contents were transmitted to large petri dishes containing the saline solution. The contents were examined by naked eye and under the dissecting microscope.
The liver of each rat was examined for the presence of cysts. Livers with Cysticercus fasciolaris parasitic larval cysts were collected in normal physiological saline. To study the morphology, the cysts were opened via a small slit to release the parasites. Larvae and other parasites found were preserved in 10% formol saline for later identification.
Parasitological Examinations
Stools examinations: Intestines were detached, separated and their contents were firmly squeezed into phosphate buffer saline (PBS). Afterwards, contents were homogenized by vigorous shaking. Saline wet mounts were made and examined microscopically.
Concentration procedures: This technique was applied to separate parasites and its eggs from fecal debris. Both types of this test, sedimentation, and floatation were applied (Stool Specimens - Specimen Processing, DPDx - Laboratory Identification of Parasites of Public Health Concern, CDC, 2016).
Perianal cellophane tape testing: A single piece of cellophane tape was tightly applied to the anal region for each rat. The tapes were attached to standard microscope slides and examined by using a 4X microscope objective (Hill et al., 2009).
Blood Examination
Blood collection: A representative number of the received rats were bled after euthanasia and necropsy from the heart directly. Blood was collected and divided into anticoagulant and plain tubes. Serum was stored at -20oC. Anticoagulated blood was centrifuged for 10 minutes at 1000 x g and plasma was stored at -20oC. All samples were kept at -80oC for future laboratory works.
Blood flagellates examination: Blood films later were stained with Giemsa. The blood samples were examined by dry and wet smears. Fresh blood from the captured rats was examined at 400X and 1000X in oil immersion for Giemsa-stained smears. Blood flagellates were identified according to Hoare, (1972).
Statistical Methodology
Descriptive statistics were performed using proportions for qualitative variable while it was the mean and deviation standard for quantitative variables. Furthermore, the chi-square test was used for proportions comparison in assessing the endoparasites and Trypanosoma infestation proportions per gender as well as per species of the live-captured rodents. Stata IC software version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.) was used for statistical analysis. P-value less than 0.05 was considered statistically significant.
Results
A total of 839 rodents were live-captured during the study period in Jeddah. The Rattus norvegicus was the most predominant with 742 (88.44%) and followed by the Rattus rattus and Mus musculus with 94 (11.20%) and 3 (0.36%) respectively. The female proportions in Rattus norvegicus were 54.58% and 56.38% in Rattus rattus species. The highest weight was reported in Rattus norvegicus species with 350 gm as median and ranged from 30 to 1000 gm. In the Rattus rattus and Mus musculus species, the median weight were respectively 150 gm (min=20; max=400) and 50 gm (min=15; max=130). The mean length for the different species was respectively 30.45, 26.07 and 24.33 for Rattus norvegicus, Rattus rattus and Mus musculus (Table 1).
Table 1: Characteristics of collected rodents
| Parameters |
Rattus norvegicus n=742 |
Rattus rattus n= 94 |
Mus musculus n=3 |
|
Gender, n (%) Male Female |
337(45.42) 405(54.58) |
41(43.62) 53(56.38) |
0(0) 3(100) |
|
Weight (gm)
Median (P25 – P75) |
350 (250 – 450) |
150 (100 - 200) |
50 (15 – 130) |
|
Length (cm) Mean (SD) |
30.45(6.80) | 26.07(4.44) | 24.33(6.42) |
Ectoparasites Identified in Collected Rodents
Overall, the ectoparasites identified during the study period were fleas 114 (13.59%), mites 43 (5.13%) and ticks 7 (0.83%). Out of 742 Rattus norvegicus live-captured, 106 (14.29%) had positive fleas, 7 (7.45%) in Rattus rattus species and only 1 out of the 3 Mus musculus species. Oppositely, the mites were predominant in Rattus rattus compared to Rattus norvegicus with 14 (14.89%) and 29 (3.91%) respectively. The ticks were found only in the Rattus norvegicus species while there were no mites neither fleas in the Mus musculus species (Table 2 & 3).
Classification of the ectoparasites was performed according to the Centers for Disease Control and Prevention (2003) key for arthropod taxonomy.
Table 2: Identified ectoparasites per live-captured rodents species
| Species | Fleas no(%) | Mites no(%) | Ticks no(%) |
|
Rattus norvegicus (n=742) |
106(14.29) | 29(3.91) | 7(0.94) |
|
Rattus rattus (n=94) |
7(7.45) | 14(14.89) | 0(0) |
|
Mus musculus (n=3) |
1(33.33) | 0(0) | 0(0) |
Table 3: Identified Fleas per live-captured rodents gender
| Species | Fleas no(%) | p-value |
| Total male rats (n=378) | 37(9.78) | 0.316 |
| Total female rats (n=461) | 68(14.75) |
Three species of fleas were identified, Xenopsylla cheopis (Oriental rat flea) (Figure 1) was the prominent species detected in rats. Most of isolated fleas were on the brown rat. Besides, we were able to identify two other species of fleas, Ctenocephalides felis (Cat flea) (Figure 2) and Echidnophaga gallinacean (Sticktight Flea) (Figure 3).Their percentages were exceedingly less than the former one.

Figure 2:Ctenocephalides felis (Cat flea)
Table 4: Distribution of endoparasites and ectoparasites per municipality
|
Municipality |
n(%) |
Ectoparasites | Endoparasites | ||
| Fleas | Mites | Ticks | n(%) | ||
| UmAssalam | 12(1.43) | 1(8.33) | 1(8.33) | 0(0) |
7(58.33) |
| Aljanoub | 36(4.29) | 4(11.11) | 0(0) | 0(0) | 6(16.67) |
| Khuzam | 37(4.41) | 8(21.62) | 1(2.70) | 0(0) | 6(16.22) |
| Aljamaa | 106(12.63) | 9(8.49) | 1(0.94) | 0(0) | 23(21.70) |
| Albalad | 220(26.22) | 28(12.73) | 9(4.09) | 5(2.27) | 51(23.18) |
| Breman | 33(3.93) | 3(9.09) | 0(0) | 0(0) | 7(21.21) |
| Alsharafyiah | 49(5.84) | 15(30.61) | 8(16.33) | 2(4.08) | 5(10.20) |
| Alazeezyiah | 82(9.77) | 14(17.07) | 5(6.10) | 0(0) | 20(24.39) |
| Jeddah Aljadeeda | 120(14.30) | 10(8.33) | 8(6.67) | 0(0) | 16(13.33) |
| Almatar | 96(11.44) | 15(15.63) | 5(5.21) | 0(0) | 24(25) |
| Ubhor | 44(5.24) | 5(11.36) | 4(9.09) | 0(0) | 6(13.64) |
| Zhahaban | 4(0.48) | 2(50) | 1(25) | 0(0) |
3(75) |

Figure 3:Echidnophaga gallinacean (Hen Flea)
The mites identified were belonging to genus Ornithonyssus spp (Tropical rat mites) (Figure 4).

Figure 4: Ornithonyssus spp (Tropical rat mites)
The number of ticks isolated was very scarce among the whole span of the study. The main species of ticks isolated was belonging to family Ixodidae (Hard ticks) (Figure 5).

Figure 5: Ixodidae (Hard ticks)
Endoparasites and Ectoparasites Distribution Per Municipality
Table 4 indicates the distribution of live-captured rodents per municipality as well as the identified ectoparasites and endoparasites. It can be seen from the table that the majority of live-captured rodents were from Aljamaa, Albalad and Jeddah Aljadeeda with 106 (12.63%), 220 (26.22%) and 120 (14.30%). In addition, UmAssalam municipality was lowest positive fleas found in live-captured rodents with 8.33% while the highest was reported in Alsharafyiah municipality with 30.61%. In the same way, Alsharafyiah municipality remained the holder of the highest positive mites whereas the lowest was documented in Aljanoub where no positive mites were found in the 36 live-captured rodents from this municipality. Furthermore, endoparasites was found in the municipality with at least one hundred live-captured rodents were respectively 21.70%, 23.18% and 16.33% for Aljamaa, Albalad and Jeddah Aljadeeda municipalities.
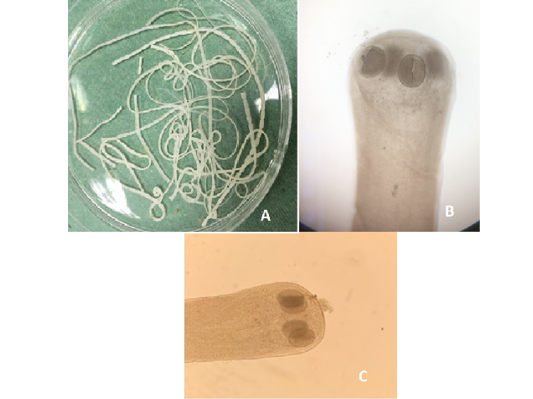
Figure 8:A) Hymenolepis nana whole worm extracted from small intestine of brown rats; B) Hymenolepis nana (Head suckers); C) Hymenolepis diminuta (Head scolex)
Identified Endoparasites
Out of the 839 live-captured rodents over the study period, a total of 174 (20.74%) was assessed with positive of endoparasites. The Cysticercus fasciolaris (Figure 6), the larval form of Taenia taeniaeformis (Figure 7a & 7b) cestode parasite of cats, was the predominant endoparasites identified with 115 (13.7%) and followed by Syphacia spp and Hymenolepis nana & Hymenolepis diminuta with respectively 68 (8.1%) and 9 (1.07%) as number of infested live-captured rodents (Figure 8a, 8b and 8c). In addition, only 4 live-captured rodents were infested with Trichuris spp. egg or whole worm (Figure 9) whereas only one was infested with trematodes.
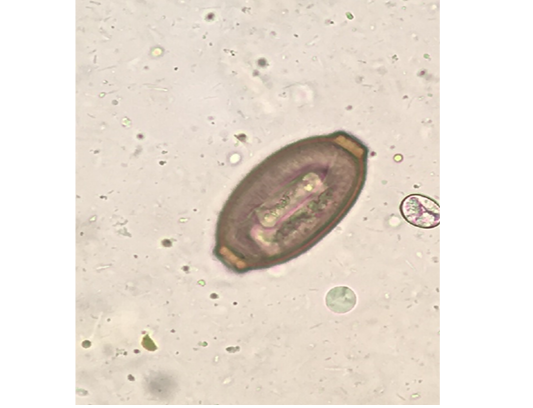
Figure 9: Trichuris spp. egg
Table 5: Distribution of endoparasites per species of rodents
| Endoparasites |
Rattus norvegicus |
Rattus rattus |
Mus musculus |
| Cysticercus fasciolaris | 111 (14.96) | 4(4.26) | 0(0) |
| Syphacia spp. | 65(8.76) | 1(1.06) | 0(0) |
|
Hymenolepis nana & Hymenolepis diminuta |
9(1.21) | 0(0) | 0(0) |
|
Trichuris spp. Egg and/or whole worm |
3(0.40) | 1(1.06) | 0(0) |
| Trematodes | 0(0) | 1(1.06) | 0(0) |
Endoparasites Distribution Per Rodent Species in Jeddah
Overall, the majority of endoparasites identified in the live-captured rodents were in the Rattus norvegicus species group (Table 5). No endoparasites were found in the Mus musculus rodent species. In addition, a total number of 111 (14.96%) of the species Rattus norvegicus were infested by Cysticercus fasciolaris and only 65 (8.76%) and 9 (1.21%) were infested respectively by Syphacia spp and Hymenolepis nana & Hymenolepis diminuta. Furthermore, the number of infested rodent in the Rattus rattus species by Cysticercus fasciolaris was 4 (4.26%) whereas 1 (1.06%) infested by trematodes, Syphacia and Trichuris spp.
Assessment of Endoparasites Per Species and Gender of Rodents (Table 6)
There was a significant difference in the positive endoparasites identified in the Rattus norvegicus compared to the Rattus rattus species with respective proportions 22.51% and 7.45% (p = 0.001). On the contrary, it can be seen from the table below that the positive endoparasites was similar in male and female with 20.37% versus 21.07% (p=0.812).
Table 6: Endoparasites per species and gender of rodents
| Parameters | n(%) | p-value |
|
Rodent spp. Rattus norvegicus Rattus rattus |
167(22.51) 7(7.45) |
0.001 |
|
Gender Male Female |
77(20.37) 97(21.07) |
0.812 |
Assessment of Trypanosomiasis per Species and Gender of Rodents (Table 7).
The overall infestation with Trypanosoma in collected and examined rats were 18.6%. There was no significant difference in the positive Trypanosomiasis identified in the Rattus norvegicus compared to the Rattus rattus species with respective proportions 18.6% and 1.11% (p < 0.808). On the other hand, there was a very high significance in Trypanosomiasis between males and females (p > 0.001).
Table 7: Trypanosomiasis per species and gender of rodents
| Parameters | n(%) | p-value |
|
Rodent spp. Rattus norvegicus Rattus rattus |
79(18.6) 5(1.11) |
0.808 |
|
Gender Male rats Female rats |
62(73.8) 22(26.2) |
> 0.001 |
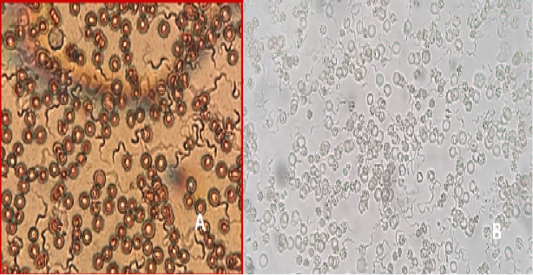
Figure 10: A) T. lewisi, whole blood film stained with Acridine orange; B) T. lewisi under Florescent microscopy
Microscopic Observations of the Blood Samples
Identification of the trypanosomes was performed by microscopic observation based on the morphological features of the parasites. The Trypanosoma spp. detected in the blood smears was suspected to be Trypanosoma lewisi. (Figure 10a & 10b). It has a long thin posterior end, with a sub terminal oval kinetoplast, the nucleus is in the anterior part of the body, and part of the flagellum is free.
Discussion
The cats are the definitive host of Taenia taeniaeformis. The larval stage of T. taeniaeformis, named Cysticercus fasciolaris, takes small rodents as intermediate host. In this study we found (Table 5) that C. fasciolaris rate in R. norvegicus was (14.96%). There was a statistical significance between brown rats and black rats infestation (p = 0.001). This significance may be referred to the behavior of the brown rats in terms of its normal habitat is outside homes and premises. This behavior gave this species a chance to be a pray to the stray cats and vice versa. Previous researchers reported prevalence in brown rats ranged from 100% in the Philippines (Claveria et al., 2005) to 29.9% in Serbia (Kataranovski et al., 2010). Variations in the prevalence of C. fasciolaris in different countries indicate risk factors of infection, including seasonal variation in the infection and pressure on the intermediate hosts (Burlet et al., 2011). Incidence of the cyst regarding to gender was reported in Table 6 and showed no statistical significance in infestation rate in terms of gender (p = 0.812). The same results were obtained by Sharma and his colleagues (2017) where they found that there was no significant statistical difference between male and female rats in prevalence of C. fasciolaris.
Hymenolepis nana and H. diminuta (tapeworm of rodents) were identified in the intestinal contents of collected rats (Table 5). This cestode showed considerable percentage (1.2%). R. norvegicus was the main host of this cestode. Same results were recorded in many countries such as Egypt (Elshazly et al., 2008), Philippines (Claveria et al., 2005), India (Singla et al., 2008) and Saudi Arabia (Al-Quraishy et al., 2014) where H. diminuta was isolated from Rattus spp. but in contrary to our results cestode was prevalent in black rats than the brown one.
Oriental rat fleas, Xenopsylla cheopis, was the main flea detected on all types of rats collected from the city. Brown rat is considered the main reservoir of this type of fleas. This flea is the primary vector of Yersinia pestis and is involved in the transmission of Rickettsia felis (Bitam et al., 2010). Rat flea was isolated from rats collected from Riyadh city (Alahmed and Al-Dawood, 2001). Two other types of fleas of animal health importance were isolated from brown rats, but with low rate of infestation. Ctenocephalides felis (Cat flea) is extremely common on cats and dogs in many temperate and tropical regions and recorded on rats (Bitam et al., 2010). Due to its ability to carry infectious agents such as Rickettsia felis hence its worldwide distribution represents a threat to the human population because of lack of host specificity and its ability to bite people (Parola, 2011). Echidnophaga gallinacea (Sticktight flea) are by no means restricted to fowl, and also infest a wide variety of mammals, including dogs, cats, rabbits, rodents and birds (Soliman et al., 2001; Bitam et al., 2010). However Alicata, (1942) was able to prove the experimental transmission of the Typhus rickettsiae from rat to rat with the Sticktight flea.
The overall prevalence of infected rats with Trypanosoma lewisi in the present study was (18.6%) which is close to percentage recorded by Linardi and Botelho, 2002. But this result is higher than that in other countries such as Egypt (Abdel-Aal & Abou-Eisha, 1997) which was 13.2%. There was no significant difference in the positive Trypanosomiasis identified in both species of rats. The high incidence could be endorsed to ecological and behavioral factors. Male rats show territorial behavior and are significantly more infested by Xenopsylla cheopis than females increasing their chances of being infected by Trypanosoma lewisi.
Little is documented on the field survey on wild rats and its related pests to stand on the volume of its annoying in Saudi Arabia. Most of the field surveys were run related to one or two specific pests. Regarding sample number; surveys did not represent the community and environment. For instance, Toxoplasmosis in Rattus rattus was investigated in the city of Riyadh, revealing positive cases with Toxoplasma gondii (Elamin, 2014). On the other hand, cestode Hymenolepis diminuta as a bio-indicator for lead accumulation was investigated in the industrial areas in the city of Riyadh (Al-Quraishy et al., 2014). However, ectoparasites took the same little attention in terms of samples diversity and number of rats collected (Asiry and Fetoh, 2014).
Conclusion
The results of this survey show that the common rat species detected in the City of Jeddah were R. norvegicus and R. rattus. Rats are considered a good carrier of diverse animal health pests comprising ectoparasites such as fleas and ticks and endoparasites such as cestodes, Hymenolpes spp. and Cystisecrcosis and blood flagellates which is Trypanosoma lewisi. Parasite reservoir studies are an important element of any integrated public health response to established or emerging diseases. Because of important role of rodents in spreading different parasitic agents and consequently bacterial, rickettsial and viral agents control programs are needed for reducing their adverse impact.
conflict of interest
The authors declare that there is no conflict of interest.
Authors contribution
Ahmad Allam and Eid Saed Ibrahim: Receiving rats, collection of samples, run all experiments and writing of the manuscript.
Adama Kazienga: made all appropriate statistics works and writings related to the manuscript.
Fahad Al Zahrani: Follow up with the Control Company and Jeddah Municipality regarding the flow of the samples and financial support.
Funding
This work was supported in part by the Project of operating and development the Public Health Pests Laboratory of Jeddah Governorate, Jeddah city, Saudi Arabia.
References








