Advances in Animal and Veterinary Sciences
Clinico-pathological and Biochemical Aspects of Foot and Mouth Disease in Calves
Saadoon Abdul-Satar Salim1, Al-Obaidi Qaes Talb2*, Al-Mahmood Saevan Saad3, Albaroodi Safwan Yousif4
1Department of animal production, College of Agriculture and Forestry, University of Mosul, Mosul, Iraq; 2Department of Internal and preventive medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq; 3Department of Pathology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq; 4Department of Microbiology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq.
Abstract | The aims of this study were to evaluate the prevalence of foot and mouth disease virus (FMDV) nonstructural protein (NSP) antibodies in calves serum using competitive enzyme linked immunosorbent assay technique (c-ELISA), to verify the clinical forms of the disease, and to asses the histopathological changes of myocardial form and some biochemical changes with regard to FMD. A total of 222 calves blood samples were collected from both sexes from various farms in the Mosul city, Iraq. Twenty clinically healthy calves were used as control. The overall seroprevalence of FMD in calves was 48.64%, clinical form was 86/222 (38.74%) and myocardial form was 22/222 (9.90%). Calves with clinical form were statistically significantly higher in prevalence than myocardial form (P< 0.05). Clinical examination of diseased calves revealed vesicles in the mouth and on their feet, with rope salivation, fever and polypnea. Other calves exhibited heart problems manifested as murmur sounds, irregular heart rhythm, tachycardia and most of these calves were recumbent, unable to suck their dams and died within 24 – 72 hrs. Pathological examination of dead calves showed presence of tiger-heart, which is the pathognomic gross lesions of acute form of FMD with severe ischemic infarction and focal myocarditis associated with infiltration of inflammatory cells mainly of mononuclear type. The biochemical profile of clinically infected calves revealed significant increase in AST, ALT, LDH, creatinine, BUN, glucose, and a significant reduction in total protein, albumen, globulin, while there was no substantial variance in the levels of total bilirubin, cTn-I, calcium, and phosphorus compared with the control group (P< 0.05). In the calves with myocardial form there was a substantial rise in the levels of LDH, BUN, cTn-I, and calcium in comparison with the control group (P<0.05). This study concluded that FMD causes clinico-pathological and biochemical changes in calves and myocarditis associated with FMD always terminates with death of infected calves.
Keywords | Clinico-pathology, Biochemical changes, Myocardial form, FMD.
Received | April 23, 2019; Accepted | July 13, 2019; Published | September 15, 2019
*Correspondence | Al-Obaidi Qaes Talb, Department of Internal and preventive medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq; Email: qaes_talb@yahoo.com
Citation | Salim SAS, Talb OQ, Saad MS, Yousif AS (2019). Clinico-pathological and biochemical aspects of foot and mouth disease in calves.Adv. Anim. Vet. Sci. 7(10): 835-843.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.10.835.843
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Talb et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Foot and mouth disease (FMD) is a extremely contagious disease of domestic and wild ruminants, is endemic in Asia and Africa and affects the animals’ productivity by way of lowered milk yield and weight loss (Parida, 2009). In non-endemic countries the disease occurs as devastating epidemics which result in very substantial economic losses through its high mortality among young animals and from the control measures that must be taken in order to attain disease-free status (Jamal and Belsham, 2013). Intentional introduction of FMD virus would be a form of agroterrorism, which may be devastating in FMD-free countries because it takes many days or much longer for the disease to be recognized for its control (Brito et al., 2017).
Foot and mouth disease is caused by the FMD virus, a small, non-enveloped virus which belongs to the genus Aphthovirus, family Picornaviridae (Belsham, 2005). The virus is present as seven major different serotypes: A, O, C, Southern African Territories SAT 1, SAT 2 , SAT 3 and Asia-1. Each serotype has multiple subtypes with variable antigenicity and different degrees of virulence, particularly within the A and O types (Constable et al., 2017).
Calves infected with FMD exhibit severe clinical signs, usually they become febrile and develop vesicular lesions on the tongue, gums, soft palate, dental pad and nostrils. Vesicles on the tongue rupture quickly and leave a raw, painful surface which heals in about one week. The animal is unable to eat, associated with profuse salivation, the saliva hanging in long rope–like strings. A typical smacking of the lips then appears and the animal chews carefully. The vesicles have a thin wall containing a straw-colored fluid (Knight and Rushton, 2013). At the same time with the oral lesions, vesicles form on the feet, in particular on the coronet and in the clefts. Rupture of feet vesicles leads to acute discomfort with severe lameness. At this stage the animal is often recumbent and there is a marked painful swelling of the coronets (Thomson and Bastos, 2004; Arzt et al., 2011).
The secondary bacterial invasion of foot lesions may prolong the healing time and may cause inflammation of the deep structures of the foot (Constable et al., 2017). Moreover, vesicles may appear on the teats and when the teat orifice is affected it leads to severe mastitis (Alexandersen et al., 2003). The mortality rate may reach 5% in adult cattle and 50% in calves due to myocardial damage, especially in young animals (Grubman and Baxt, 2004).
In suckling calves the myocarditis is a fatal form of FMD, which happens with no evidence of vesicular lesions observed in adult cattle. It is distinguished by hyaline degeneration, necrosis of muscle fibers and a high infiltration of lymphocytes (Aktas et al., 2015).
Diagnosis of myocardial disease in cattle is by physical examination, cardiac auscultation, and high incidence of sudden death in suckling calves (Dawood and Alsaad, 2018). A higher incidence of myocarditis due to FMD is more prominent in young calves compared to adult cattle (Gunes et al., 2005, Gulbahar et al., 2007, Karapinar et al., 2010). However, the solid-phase competitive ELISA has been documented to be of greater sensitivity and more rapid than the virus neutralization test (VNT) for detection of FMDV antibodies (Brocchi et al., 2006; Sevik and Ozturk, 2013).
In Iraq, FMD is an endemic disease causing a large animal health problems and a high economic losses (Mahdi, 2010). However, no study has focused on evaluating the contributing factors of myocarditis in suckling calves and age of mortality risk. Therefore, this paper studied the prevalence of FMDV nonstructural protein (NSP) in calves, to determine the clinical forms of the disease and to asses the myocardial histopathological changes and some biochemical parameters changes with regard to FMD.
MATERIALS AND METHODS
Animals and Samples Collection
From September 2017 to September 2018, this study examined a total of 222 calves were obtained from different regions with different management systems including Gogjalee, Kolantapa and Bartilla in Mosul city, Iraq, with both sexes aged between ≤ 6 months and 2 years and all these calves were no vaccination history against FMDV. Complete clinical examination was carried out for all the calves. Twenty clinically healthy calves which were negative for FMDV nonstructural protein (NSP) served as the control group. Five-milliliter blood samples were extracted from the jugular vein using 18G needle and kept in sterile vacutainer tubes with no anti-coagulant undisturbed for few minutes. The tubes were centrifuged at 2500 rpm for 15 min, serum was separated and stored at -20°C until laboratory analysis (Nasr El-Deen et al., 2017).
Competitive Enzyme Linked Immunosorbent Assay (c-ELISA)
A commercial c-ELISA kit (IDvet, Grabels-France) was used to detect anti-FMDV non-structural protein (NSP) antibodies in the serum of calves. The procedure was carried out according to the manufacturer’s instructions and by using automatic plate reader (BioTek® Elx800, USA). For each samples, calculate the competition percentage (S/N%):
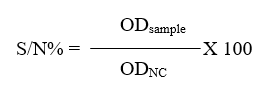
The interpretation of the results included: if samples presenting (S/N%) were less than or equal to 50% they were considered positive, while samples greater than 50% they were considered negative.
Gross Post Mortem Examination and Histopathological Techniques
The samples of heart from calves that died from an acute form of FMD were submitted for gross examination that started from epicardium and extended through myocardium to endocardium and any gross lesions were recorded with a digital camera. Later, tissue samples were collected from grossly appeared lesions on heart at thickness less than one centimeter and fixed in 10% neutral buffered formalin for 72 hours followed by dehydration with alcohol, cleared by xylol, embedded and subjected to infiltrated with hot paraffin wax, following which these paraffin embedded samples were sectioned at 6 µm (Suvarna et al., 2013). A routine Harris hematoxylin and yellow alcoholic eosin were applied to all sections for routine examination. Later, a special histochemical stain was applied to specifically selected tissue slides, which included Masson’s trichrome stain and McManus’ method period acid-schiff’s reagent stain (PAS) (Luna, 1968).
Biochemical Analysis
Serum samples of diseased calves were utilized for biochemical analysis, including aspartate amino transferase (AST), alanine amino transferase (ALT), lactate dehydrogenase (LDH), creatinine, total protein, albumins, globulins, total bilirubin, blood urea nitrogen (BUN), glucose, calcium, phosphorous, by the use of special cassettes for each in a chemistry analyzer (IDEXX- Vet Test, Arachem/USA). A total of 40μl of serum samples was used to carry out the test and the procedure was done according to the chemistry analyzer system guide book. The cardiac biomarker, Troponin-I (cTn-I) was estimated in the serum of diseased calves with a commercially available cTn-I enzyme-linked immunosorbent assay (ELISA) kit (EAGLE, Biosciences, Italy).The ELISA was conducted and the results were calculated according to the manufacturers instructions.
Statistical Analysis
The differences in the seroprevalence between the various calves’ health status were evaluated using two-sided Chi-square in IBM-SPSS statistics version 19 program. Furthermore, One way analysis of variance (ANOVA) was followed by post hoc test (Duncan) in the same program, and used to make a comparison between clinical form, myocardial form and healthy calves (control group), in serum biochemical parameters. All the important variances were determined at P< 0.05.
RESULTS
The overall seroprevalence of FMD in calves was 108/222 (48.64%) with the 95% confidence interval, comprising clinical form, which was 86/222 (38.74%) and myocardial form was 22/222 (9.90%). Calves with clinical form were statistically significantly higher in prevalence compared to myocardial form (P< 0.05) (Table 1).
The clinical examination of the infected calves reveled that the animals were suffering from depression, anorexia, fever ≥40.5°C and vesicular lesions which appeared in the buccal cavity, tongue and feet associated with rope salivation, lameness, rough hair and mouth breathing with panting. Some other diseased calves showed neither oral vesicle, rope salivation, nor lesions of interdigital space and coronary band regions, but they had heart problems reflected as murmur sounds, irregular heart rhythm, tachycardia on auscultation of the chest and most of these calves were recumbent, unable to suck their dams and died within 24 – 72 hrs, with the mortality rate at 9.90%. The various frequencies and percentages of each clinical sign were recorded in calves infected with FMD (P< 0.05) (Tables 1, 2). Moreover, statistically significant rise in the body temperature, heart rate and respiratory rate in the calves with the clinical form and myocardial form compared with control group. Abnormal sound (organic murmurs) was auscultated during the examination of the chest in the calves with myocardial form only (Table 3).
Table 1: The seroprevalence of clinical and myocardial forms of FMD in calves using c-ELISA test.
| Animal status | No. of samples tested | No. of positive samples | Percentage (%) |
| Clinical form | 222 | 86 |
38.74a |
| Myocardial form | 22 |
9.90b |
|
| Total | 108 | 48.64 |
Values significantly different (P<0.05) between the animal status are labeled with different letters (a or b).
Table 2: Frequency and percentage of clinical signs in naturally-infected calves with foot and mouth disease (108 Cases).
| Clinical signs | Frequency of signs | Percentage (%) |
| Depression | 73 | 67.59 |
| Anorexia | 98 | 90.74 |
| Fever | 103 | 95.37 |
| Lesions in the mouth | 75 | 69.44 |
| Lesions in the feet | 53 | 49.07 |
| Lesions in the mouth & feet | 48 | 44.44 |
| Rope salivation | 79 | 73.14 |
| Lameness | 66 | 61,11 |
| Rough hair | 93 | 86.11 |
| Mouth breathing and panting | 55 | 50.92 |
| Tachycardia | 108 | 100 |
| Murmur sounds | 22 | 20.37 |
| Irregular heart rhythm | 22 | 20.37 |
| Recumbency | 33 | 30.55 |
| Unable to suck their dams | 20 | 18.51 |
The results of gross pathological examination showed the presence of pathognomic lesion known as “Tiger-heart,”
Table 3: The clinical symptoms in calves with clinical form and myocardial form compared with control group.
| Parameters | Healthy calves (Control group) mean ± S.E (n= 20) | Clinical form Mean ± S.E (n =86) | Myocardial form Mean ± S.E (n = 22) |
| Body temperature/ ºC |
38.5 ± 0.38a |
40.6 ± 0.7 b |
41.2 ± 0.5b |
| Heart rate / min |
91.4 ± 2.42 a |
116.2± 3.6 b |
122.1 ± 4.2b |
| Respiratory rate / min |
29.4 ± 4.5 a |
41 ± 4.2 b |
68.3 ± 3.4c |
|
Gallop rhythm (organic murmurs) |
_ | _ |
+ |
Mean values ± Standard error (S.E.) significantly different (P < 0.05) between calves healthy status are labeled with different superscript letters (a, b or c).
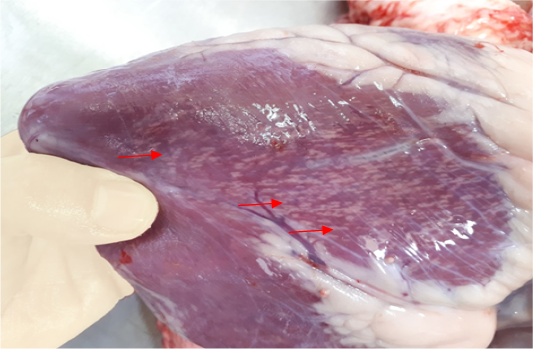
Figure 1: Gross image of calf’s heart shows pathognomic gross lesions termed as “tiger-heart” (arrows)

Figure 2: Microscopic image of calf’s’ heart (Tigroid heart appearance) from calf that died from an acute form of FMD, showing myocarditis represented by focal coagulative necrosis of muscle fibers (arrow), with interstitial infiltration of mononuclear inflammatory cells (arrowhead). (H&E, 100x.).
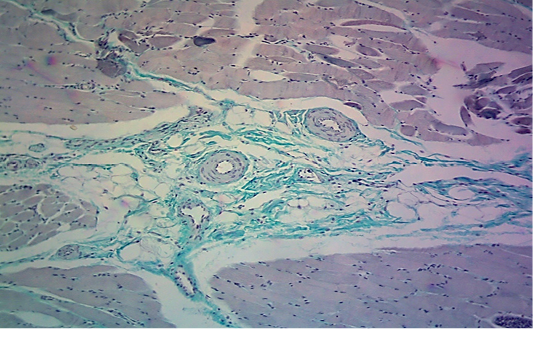
seen as gray streaks on the epicardium (Figure 1). These gross lesions appear due to focal degeneration and necrosis of myocardial fiber, which leads to interstitial infiltration of mononuclear inflammatory cells (Figure 2), while lymphocytes were the predominating infiltrated cells with a few plasma cells and histocytes (Figure 3). These infiltrated cells are associated and embedded with collagen deposition that is mostly seen around the blood vessels (Figure 4), and these degenerated and necrotic myocardial fibers were reacted strongly positive with Schiff’s regent in PAS techniques (Figure 5).
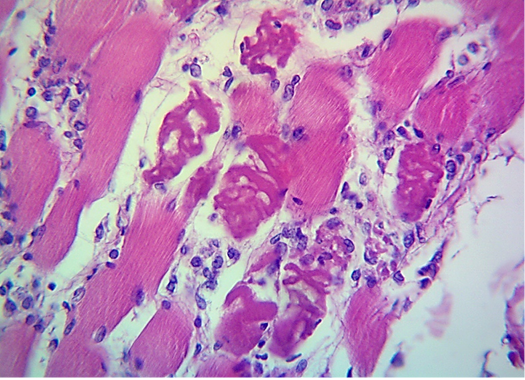
Figure 3: Microscopic image of calf’s’ heart (Tigroid heart appearance) from calf that died from an acute form of FMD, showing focal coagulative necrosis of muscle fibers (arrow) with interstitial infiltration of lymphocytes, plasma cells and histocytes (arrowhead). (H&E, 600x.).
Table 4: Serum biochemistry parameters in calves infected with foot and mouth disease (FMD) and healthy calves. Data are presented as mean ± standard error of mean.
| Parameters | Healthy calves (Control group) Mean ± S.E (n= 20) | Clinical form Mean ± S.E (n =86) | Myocardial form Mean ± S.E (n = 22) |
| AST U/L |
58.33 ± 17.46a |
98.23 ± 19.12b |
62.52 ± 12.22 a |
| ALT U/L |
36.23 ± 11.45a |
52.18 ± 9.43b |
41.92 ± 7.76 a |
| LDH U/L |
121.12 ± 37.71 a |
292.23 ± 18.23b |
241.12 ± 27.71b |
| Creatinine mg/dl |
0.92 ± 0.13 a |
1.95 ± 0.22b |
1.01 ± 0.07a |
| Total protein g /dl |
7.57 ± 0.66a |
5.12 ± 0.24b |
7.02 ± 0.14 a |
| Albumin g/dl |
3.66 ± 0.65a |
2.47 ± 0.30b |
3.42 ± 0.65a |
| Globulin g/dl |
3.83 ± 0.21a |
3.02± 0.13b |
3.33 ± 0.21a |
| Total bilirubin mg/dl |
0.42 ± 0.10a |
0.38 ± 0.02a |
0.40 ± 0.07a |
| BUN mg/dl |
16.55 ± 7.12a |
27.34 ± 5.76b |
28.16 ± 6.46b |
|
Cardiac Troponin-I (cTn-I) ng/mL |
0.34 ± 0.06a |
0.41 ±0.04 a |
15.65 ± 1.8b |
| Glucose mg/dl |
56.76 ± 14.66a |
98.41 ± 9.87b |
58.16 ± 8.36a |
| Calcium mg/dl |
9.32 ±1.84a |
9.80 ±0.92a |
10.78 ±1.02b |
| Phosphorous mg/dl |
5.13 ±1.22a |
5.22 ±1.72 a |
5.52 ±1.33a |
Mean values ± Standard error (S.E.) significantly different (P < 0.05) between calves,
and those of healthy status are labeled with different superscript letters (a, b or c).
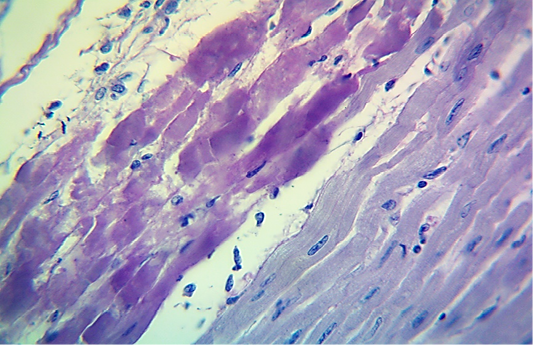
Figure 5: Microscopic image of calf’s heart (“tiger-heart”) from a calf that died from an acute form of FMD, showing severely affected muscle fibers with coagulative necrosis that stained magenta in color (arrow), with infiltration of lymphocytes (arrowhead). (PAS stain, 600x).
Serum biochemistry analysis of the calves with clinical form showed a considerable increase in AST, ALT, LDH, creatinine, BUN, glucose and a significant decrease in total protein, albumen, globulin, while no significant difference was evident in the levels of total bilirubin , cTn-I, calcium and phosphorus compared with the control group (P< 0.05). In the calves with myocardial form a significant increase was detected in the levels of LDH, BUN, cTn-I and calcium, while there was no significant difference in the levels of AST, ALT, creatinine, total protein, albumen, globulin, total bilirubin, glucose and phosphorus compared with the control group (P < 0.05) (Table 4).
Discussion
FMD is endemic in Iraq, appearing periodically and causing major economic losses in Iraq. In the present study the overall seroprevalence of FMD in calves was 48.64% in Mosul city, a result that is lower in comparison with earlier studies in Iraq. Abd-Alhameed and Rhaymah (2010a) reported that the seroprevalence of FMD in calves in Mosul city was 91.5%, using competitive ELISA test. The seroprevalence of FMD in cattle in Basrah governorate, Iraq was 94.5% (Al-Rodhan, 2014). In central and south of Iraq the result was 61.42% using FMD 3ABC Bo Ov ELISA test (Abood et al., 2009). The seroprevalence of FMD among cattle in Karbala and Al-Najaf in Iraq were 100%, and 75% respectively (Al-Budeiri, 2012). The seropositive findings in our study are higher than the overall seroprevalence of the disease in cattle in Al-Diwaniyah and Diyala provinces in Iraq which were 34.09% and 25.33% respectively (AL-Jobori, 2012, Al-Ajeeli et al., 2018). Studies undertaken in other countries reported different prevalence of FMD in cattle such as: 16% in Saudi Arabia, 13.5% in Central Ethiopia, 31% among Egyptian calves, 55.2% in Northwestern Nigeria (Hafez et al., 1994, Alemayahu et al., 2014, Babangida et al., 2017, Hefnawy et al., 2018). The prevalence of FMD varies from one country to another and between regions within the same country, which might be due to different management practices, the sensitivity and specificity of the diagnostic tests, the serotypes of this virus, the breeds of the calves, existence and efficiency of regulatory programs and the seasons and climatic differences (Abbas et al., 2012, Babangida et al., 2017, Al-Ajeeli et al., 2018).
This study demonstrated that the mortality rate in young calves is about 9.90%, a finding that is compatible with the results of Aktas et al. (2015), who revealed that the mortality rate was 11.33% in FMD-infected calves due to the myocardial form of the disease. On the other hand, Aidaros (2002) reported that 50.678 heads of cattle had been infected with the FMD virus in 13 governorates of Iraq with a mortality rate of 3.830 of cattle (7.5%). In 1998, an FMD outbreak was reported in Iraq which affected 25% of the bovine population and more than 500.000 newborn animals died because FMD virus had high affinity for myocardial muscles which leads to acute myocardial damage and terminating in death of the infected animals due to acute heart failure (El-Idrissi, 2003).
The current study showed that diseased calves with the clinical form of suffering from depression, anorexia, fever, and development of vesicular lesions in the mouth and on the feet associated with profuse drooling salivation and lameness, were the same signs as those indicated by Alexandersen et al. (2003), Abd-Alhameed and Rhaymah (2010b) and
Constable et al. (2017). Other diseased calves with the myocardial form showed neither oral vesicles, rope salivation nor lesions of interdigital space and coronary band regions, but they had heart problems. These results concur with the findings of many other earlier researchers (Aktas et al., 2015; Pinto, 2017, Hefnawy et al., 2018).
In young calves, FMD virus has a high affinity for myocardial muscles and cardiac muscles infection frequently causes death due to acute myocardial damage and acute heart failure (Alexandersen et al., 2003; Constable et al., 2017). Regarding the pathogenesis of the disease, FMD virus receptors, as well as other Picornavirus receptors, may have an important part in tissue and organ tropism (Schneider, 2000). The entry of FMD virus in to the heart cells in vivo is suspected to involve the attachment of the RGD loop of VP1 on the viral capsid to the host integrins, such as αvβ1, αvβ3, αvβ5,or αvβ6 on the surface of target cell (Jackson et al., 2000; Jackson et al., 2002). All myocarditis cases of FMD reported in this study were under 6 months of age, and this finding is in agreement with Burgess et al. (2001) who mentioned that the age associated changes occur in the cardiac matrix and integrins of the heart. Till now, many myocarditis cases due to FMD have been documented in one week old to six months old calves (Gulbahar et al., 2007; Karapinar et al., 2010; Aslani et al., 2013; Kaya et al., 2013).
The results of the pathological investigation obtained in the current study showed similar lesions in the heart as reported in other studies which included gross “tiger-heart” appearance with severe microscopic ischemic infraction and focal myocarditis with infiltration of inflammatory cells (Rigden et al., 2002; Alexandersen and Mowat, 2005; Knowles et al., 2005). In the acute form of FMD, the virus replication in affected myocardial fibers will lead to degeneration and necrosis of these fibers (McGavin and Zachary, 2007), which later will develop to be ischemic infraction of cardiac muscle at focal locations (McGavin et al., 2001), and this will lead to stoppage of electrical signal transport to other parts of the cardiac muscle leading to complete heart failure (Aktas et al., 2015, Islam et al., 2017). In autopsy, variable sizes of pale foci appear on the ventricular muscles which are referred as “Tiger-heart” and these gross lesions are similer to other lesions which develop in many other syndromes of acute myocardial damage, and the necrosis of fibers may be striking (Kostin et al., 2003). The animals that survive after the acute phase of FMD infection may develop chronic lesions such as myocarditis, necrosis and scarring, heat intolerance, pancreatitis with acinar necrosis and regeneration (Jamal and Belsham, 2013). The histopathological changes in the cardiac tissues of the calves with myocarditis revealed the presence of vacuolated , degenerated myocardial muscle cells associated with interstitial edema and intense mononuclear cell infiltration, hyaline degeneration and necrosis which are compatible with the findings of previous studies performed by Gunes et al. (2005) and Karapinar et al. (2010).
This current study found that the serum biochemical analysis of calves clinically-infected with FMD showed a substantial increase in AST, ALT, LDH and creatinine, which could be attributed to the degenerative changes of harmful effects of FMD virus on the liver and heart and/or to hepatocellular damage, findings which are in agreement with evidence in the literature (Nath et al., 2014; Nasr El-Deen et al., 2017). This is in contrast to Ghanem and Abdel-Hamid (2010) who reported no significant changes in the levels of AST, ALT, LDH in cattle clinically- infected with FMD. This difference may be due to difference in the serotypes of the FMD virus that resulted in a substantial reduction in the serum levels of total protein, albumin, and globulin in the clinically-infected calves. This hypoproteinaemia may be due to hypoalbuminaemia and hypoglobulinaemia which occurs because of the decreased feed intake as a result of loss of appetite, pain in the buccal cavity as a result of the presence of lesions in the mouth, and also the decrease in the synthesis of proteins in the liver as a result of liver damage, mal-digestion and absorption of proteins due to the lesions in the gastrointestinal tracts and loss of plasma through the exudation of plasma from ulcers (Nasr El-Deen, 2013). Moreover, there is a noticeable increase in the level of glucose in the clinically-infected calves, a result that is compatible with Mousa and Galal (2013) and Nasr El-Deen et al. (2017). Hyperglycemia is probably due to low insulin level in the serum due to replication of FMD virus in the pancreas which leads to destruction of insulin producing cells (beta-cells in pancreas), and is in contrast to Krupakaran et al. (2009) who reported a significant reduction in blood glucose level in FMD-infected cows, this difference possibly due to the difference in animal breeds.
The present study showed a significant increase in the level of serum cardiac Troponin–I (cTn – I) and LDH in the myocardial-infected calves in comparison with the control group, and this result concurs with Leonardi et al. (2008), Tunca et al. (2008), Aktas et al. (2015), and Dawood and Alsaad (2018). Determination of specific cardiac biomarkers is very important for the diagnosis of myocarditis in animals such as serum cardiac troponins measured in humans and various animal species including dogs, cats, horses, sheep, goats, and cattle as indicators of myocardial injury (Constable et al., 2017). There are many diseases which cause increase in the concentration of serum cardiac troponin like vitamin E and selenium deficiency, traumatic reticuloperitonitis, idiopathic pericarditis and FMD in calves (Gunes et al., 2005). A considerable rise in the level of BUN in the myocardial-infected calves in comparison with the control group of animals is probably due to the harmful effects of FMD virus on the renal cells (Constable et al., 2017).
In conclusion, this study indicates that vesicular lesions and pathognomic clinical signs of FMD are not evident in myocardial form of FMD in young animals and the elevation of serum cardiac troponin (c-TnI) is a highly sensitive test for detection of myocardial cell damage in the initial stages of the disease and the risk of myocarditis related to FMD virus is associated with the age of the animals, being higher in calves younger than 6 months of age.
ACKNOWLEDGEMENTS
The authors wish to thank the College of Veterinary Medicine for the financial support of surveillance work, the expert technical assistance from veterinary clinical pathology, laboratory of veterinary teaching hospital, Staff of the department of internal and preventive Medicine for their support and the calves’ owners for their cooperation.
CONFLICT OF INTEREST
Authors declare no conflicts of interest of the manuscript.
AUTHORS’ CONTRIBUTION
All authors contributed substantially to this study and are in full agreement with the content of the manuscript.
REFERENCES