Advances in Animal and Veterinary Sciences
Research Article
Effect of Ketoconazole on Early Maternal Separation Stress Model in Rats: A Neurobehavioral and Biochemical Approach
Nawel Attoui¹, Kamilia Guedri²*, Khadidja Makhloufi¹
¹Department of biology, University Tahri-Mohamed, Béchar, 08000, Algeria; 2Department of biology, University Larbi Tebessi, Tebessa, 12000, Algeria.
Abstract | Adverse early life events, such as early maternal separation, may alter the normal pattern of brain development and subsequently the vulnerability to a variety of mental disorders in adulthood. Maternal separation relatively changes behavioral and biochemical responses leading to an overactivation of the hypothalamic–pituitary–adrenal (HPA) axis, and subsequently increases the ACTH level. In this study, we aimed to evaluate the role of a corticosteroid blocker, ketoconazole, in behavioral, hematological and biochemical changes in rat underwent maternal separation (MS). For this purpose, On postnatal days 1–7, rats were submitted daily to MS for 1h30min per day. At 3 months of age, rats were given a high dose of ketoconazole (200mg/kg), exploratory activity and anxiety-like behavior were evaluated using the open field test and elevated plus maze test, olfactory-like behavior was evaluated using the olfactory recogneting and biochemical and hematological profiles were also evaluated. We found that MS provoked depressive and anxiety-like behaviors, olfactory sensibility and biochemical changes (increase in level of blood glucose and ACTH) in adult rat. In addition, treatment of stressed rat by ketoconazole could modulate the above-mentioned negative effects of maternal separation“MS”. In conclusion, our results demonstrated that ketoconazole decrease anxiety like behavior and regulate biochemical change induced by maternal separation stress.
Keywords | Maternal Separation, Ketoconazole, Behavior, ACTH, Rat.
Received | April 09, 2018; Accepted | July 14, 2019; Published | August 25, 2019
*Correspondence | Kamilia Guedri, Department of Biology, University Larbi Tebessi, Tebessa, 12000, Algeria; Email: guedrikamilia@yahoo.fr
Citation | Attoui N, Guedri K, Makhloufi K (2019). Effect of ketoconazole on early maternal separation stress model in rats: a neurobehavioral and biochemical approach. Adv. Anim. Vet. Sci. 7(9): 761-769.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.9.761.769
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Guedri et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Social ties, principally mother-infant relationship during the infantile period, have a critical role in the development of the brain and behavior in adulthood (Amiri et al., 2016). It is shown that neonate exposure into psychosocial adversities, like maternal separation (MS) stress, dramatically interrupt brain development and augments the risk of neuropsychiatric diseases including anxiety and depression (Haj-Mirzaian et al., 2017).
The maternally separated (MS) rat is a well-established and validated model of early life stress (O’Mahony et al., 2009; Plotsky and Meaney 1993; Wigger and Neumann 1999).
Maternal separation (MS) is defined as a deficiency of proper maternal care and guidance. It includes permanent farewell and short-term separation or repetitive separation during the process of early childhood development. MS can affect the development of an individual’s emotion, cognition and social behaviors (Li et al., 2013). Clinical studies suggest that adults with the experience of childhood parental loss are vulnerable to stress and have a greater risk of suffering psychiatric disorders such as depression and anxiety (Longstaffe, 1979; Harris et al., 1986; Agid et al., 1999).
Separation from the dam during a crucial neonatal period instigates developmental changes in the central nervous system (Plotsky et al., 2005) that result in alterations in hypothalamic–pituitary–adrenal (HPA) axis regulation (Lippmann et al., 2007) in adulthood. Previous functional studies from our laboratory have demonstrated these rats exhibit increased anxiety and stress-induced defecation in the open field arena (O’Malley et al., 2010a, Melo et al., 2018) and increased pain sensitivity in response to colorectal distension (Hyland et al. 2009; O’Mahony et al., 2008). Recently, it has been determined that MS via modulation of oxytocinergic system increased pain sensitivity (Amini-Khoeia et al., 2019). CRF is crucial to the regulation of anxiety and depression (Bale and Vale, 2004; Zorrilla and Koob, 2004). and mediates stress-induced changes in GI function (Williams et al., 1987), relating alterations in receptor expression to phenotypic changes should provide a better understanding of the complex interactions between stress susceptibility and disease. Hyperactivity of HPA axis by stress-induced important secretion of catecholamine and glucocorticoid which are believed to underlie the onset of many physiological changes, among these: the psychiatric disorders.
Indeed, increased expression and stress-induced secretion of hypothalamic CRF (Kuhn and Schanberg, 1998) and alterations in the expression of colonic CRF receptors (O’Malley et al., 2010b) have previously been observed in MS rats. However, CRF and CRF receptors are also expressed in extra-hypothalamic brain areas which also contribute to the manifestation of the stress response (Reul and Holsboer, 2002; Van Pett et al., 2000).
Exposure to early life stress is a major pathological contributor to the development of depression in susceptible individuals. Maternal separation (MS, removal of the dam from the litter for 3 h/day from postnatal day (PDN 2–14) is a well-established rodent model of depression (Dimatelis et al., 2012; James et al., 2014) that induces a blunted response to stress (Daniels et al., 2004) and monoaminergic disturbances comparable to those found in major depression (Amos-Kroohs et al., 2016; Ohta et al., 2014). It has been reported that ketoconazole plays an antidepressant role in the forced swimming test (FST) in rats (Frih et al., 2010; Guedri and Frih, 2015).
Ketoconazole (KTCZ), an imidazole derivative, is an antimycotic agent and a potent inhibitor of gonadal and adrenal steroidogenesis. KTCZ inhibits the synthesis of ergosterol in fungi and of cholesterol in mammalian cells by blocking 14-demethylation of lanosterol. KTCZ also interferes with several cytochrome P-450-dependent enzymes involved in steroid synthesis. It has been used successfully as a palliative treatment of Cushing’s syndrome due to its ability to lower cortisol production. High dose (24 mg/kg) acute KTCZ significantly increased plasma ACTH levels in normal rats (Burrin et al., 1986).
This increase was accompanied by a decrease in corticosterone levels confirming previous observations in man that high dose KTCZ therapy inhibits adrenal steroid production; the fall in corticosterone levels removes negative feedback to the hypothalamus-pituitary and stimulates increased ACTH secretion. Low dose (6 mg/kg) KTCZ therapy had no significant effects on ACTH secretion or adrenal steroid output in rats (Burrin et al., 1986).
The aim of this study was to explore the effects of KTCZ blockade of adrenal steroidogenesis on a model of early life stress, maternal separation, in the rat. Amelioration of behaviors disorders after reduction of corticosterone by KTCZ would suggest that elevation of corticosterone precede symptoms. This experiment tested the hypothesis that high dose of KTCZ could prevent behavioral disorders and biochemical alterations after maternal separation.
MATERIAL AND METHODS
Experimental Animals
In the present study, we used Wistar rats (Rattus rattus) obtained from the Pasteur Institute (Algiers, Algeria). The animals were housed in specific cages maintained in natural photoperiod temperature with standard conditions: an average temperature of 22 ± 4 º C and a relative humidity of 50-70%. After a twoo weeks of adaptation, 5 female rats were housed in pairs with a male for 6 days for mating, after-parturition.
Experimental Design
Twenty Offspring of three monthes age, were randomly divided into three groups, Ten rats for each.
(1) Control group (intraperitoneal; IP injected with distilled water), (2) , Group subjected to 90 min maternal separation (MS) for 7 days, (3) MS animals treated by oral gavage of ketoconazole (200mg/kg) daily for 5 days and Group maternal separation treated by ketoconazole (MS+KTCZ).
METHODS
Maternal Separation (MS)
Maternal separation (MS) consisted of removing the mother from the cage for 90 min while leaving the pups undisturbed. During the separation period, the pups were housed in a different room to disallow communication with their mothers by ultrasound vocalizations (Hofer et al., 1994). The MS group was separated from their mothers daily, on postnatal days 1–7 (PND 1–7) from 90 min.
The control animals were left completely undisturbed throughout this period, except during cage cleaning twice a week, when the animals were also weighed, and for the daily refill of food and water.
Ketoconazole treatment: Ketoconazole (KTCZ), is an agent antimycotic and a potential inhibitor of adrenal and gonadal steroidogenesis (Santen et al., 1983), after 3 months rats were treated by a high dose 200 mg/kg for 5 days by gavage (Amaral and Nunes Junior, 2008).
Behavioral Analysis
After the adaptation of 03 months, the behavioral tests were done. The innate anxiety behavior is a fundamental component of the overall behavior of rodents is manifested by the elevation of the animal to fear when placed under prior experience in an aversive environment. This behavior can be assessed using validated experimental devices, the most used are: the open field test (OF), elevated place maze test (EPM) and olfactory recogneting test (ORT).
Open Field Test (OF): The OFT was used to evaluate the locomotion of animals in response to AW and different treatments according to the criteria described by Amiri et al. (2015). The openfield apparatus was white opaque Plexiglas (50 cm x50 cm x 30 cm), which was dimly illuminated. Each mouse was placed gently on the center square (30 cm x 30 cm), and behaviors were recorded by a camera for 5 min and were analyzed. The surface of the apparatus was cleaned with 70% ethanol after each experiment.
Elevated Place Maze Test (EPM): The elevated plus maze (EPM), which is based on a natural aversion to the exploration of elevated and open areas, is used to assess exploration, anxiety and motor behavior, as well as spontaneous exploration of new environments, in rodents (Rodgers and Dalvi, 1997; Cao et al., 2016). The EPM used in the present study was made of white wood and consisted of a central square (10×10 cm) from which four arms (10×50 cm each) radiated outward, two of them with walls around the edge 40 cm high (closed arms), and two of them without walls (open arms). The arms were arranged so that the two open arms were opposite each other. The EPM was elevated to a height of 55 cm off the floor. The apparatus was placed in a dedicated room and surrounded with black curtains to reduce environmental cues. Each animal was placed in the center of the maze with its head facing one of the open arms and, upon release, was allowed to move freely throughout the maze for 5 min while its behavior was recorded with a video camera for further analysis. The apparatus was cleaned with 10% ethanol before each animal trial to minimize olfactory cues.
Olfactory recognition test (ORT): This test allows us to evaluate the ability of individuals to recognize and move towards familiar smells. They are performed in a T-maze consisting on a central branch
and two lateral branches closed by experimental enclosures. These are equipped with removable and perforated doors allowing the diffusion of the odorous molecules. The rats are subjected to the odor coming from an enclosure 45 ± 5g of clean sawdust and the other side also contains 45 ± 5g sawdust nest (clean sawdust X sawdust nest) the rat remains in labyrinth 05 min and between each pass the device will be cleaned with alcohol (Amiri et al., 2016).
Hematological Assay
Blood samples were collected from a retro-orbital puncture in heparin tubes for the determination of CBC (Complete blood count) by Hematology Analyzer (Abacus 380, 19 parameters).
Biochemical Analysis
At the end of the experimental period, animals were fasted for 8–12 h before blood collection in order to cause no interference in the analysis of blood glucose.
Blood samples were obtained after decapitation. Blood glucose was measured by one touch electronic glucometer ACU check, with an accuracy of 7.97±0.35 and and a precision p= 0.9044 (Owiredu et al., 2009). Other blood samples were collected and allowed to coagulate at room temperature for 30min and were subsequently centrifuged at 3000 tours for 10min. Serum was removed and stored at −80°C until analysis for the dosage of ACTH.
ACTH Assay
Blood samples were collected and plasma was separated using cold centrifugation. Plasma ACTH level was assayed by ACTH ELISA kit (BIOMERICA, INC. 1533 Monrovia Avenue Newport Beach, CA 92663 U.S.A) according to the manufacturer instructions.
Statistical Analysis
MINITAB (Minitab® 13.31) was used for statistical analysis. All values are presented as mean ± S.E.M. Statistical significance was evaluated by one-way analysis of variance (ANOVA) test. Significance was measured using Fisher’s least significant for the exact P values and significant differences are noted in the results. The difference between groups was considered significant when α<0.05.
RESULTS
Hematological Study
Our data (Table 1) showed that maternal separation cause a significant increase in withe blood cells and monocyte counts, and a significant decrease in neutrophils, lymphocyte, and eosinophils counts as compared to control group.
The hematological parameters results in maternal separation treated group were similar to the control group.
Table 1: Variation of hematological parameters in control, maternal separation “MS” and maternal separation treated groups “MS+KTCZ”
| Groups | Control | MS | KTCZ+MS |
|
WBC (103/ul) |
7.28± 0.29 | 14.04±0.37*** | 7.53±0.57 |
| Neurtophills (%) | 24.55±0.79 | 19.72±2.71*** | 25.02±2.11 |
| Lymphocytes (%) | 77.28±0.2 | 59.08±0.22*** | 75.25±0.12 |
| Monocytes (%) | 0.53±1.36 | 3.85±1.26*** | 0.48±0.92 |
| Eosinophills (%) | 1.73±0.20 | 0.0±0.12*** | 1.95±0.09 |
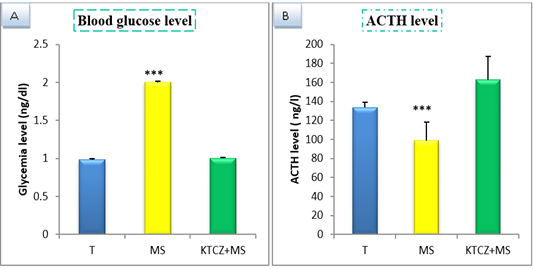
Figure 1: Change of biochimical parameters in controls, maternal separation and maternal separation treated groups A. Blood glucose level (ng/dl) b. ACTH plasmatique level (ng/l) B. (T: Temoin, MS: maternal separation, KTCZ: ketoconazole, * p < 0.05;**p < 0.01; ***p < 0.001).

Figure 2: Changes in the parameters of olfactory recognition test following control, maternal separation and maternal separation treated groups. (T: Temoin, MS: maternal separation, KTCZ: ketoconazole, * p < 0.05;**p < 0.01; ***p < 0.001).
Biochemical Study
Our results show (Figure 1) that early maternal separation results in a significant increase in Blood glucose and ACTH level compared to the control group. Treatment of stressed group by ketoconazole reduces the level of Blood glucose and ACTH.
Behavioral Study
Olfactory like behavior: Our results (Figure 2) indicate that, in maternal separation rats, the latency time for choice were increased significantly and they spent more time in center and in own nest fissure that in nest fissure.No difference significant in stressed treated groups compared to control.
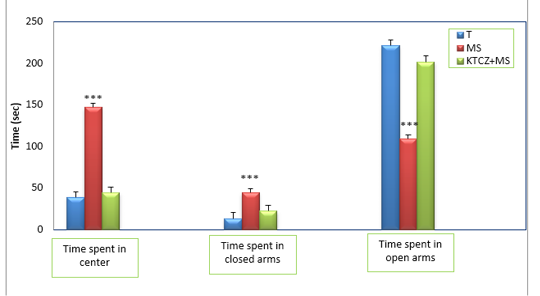
Figure 3: Changes in the parameters of the EPM test following control, maternal separation and maternal separation treated groups. (T: Temoin, MS: maternal separation, KTCZ: ketoconazole, * p < 0.05;**p < 0.01; ***p < 0.001).
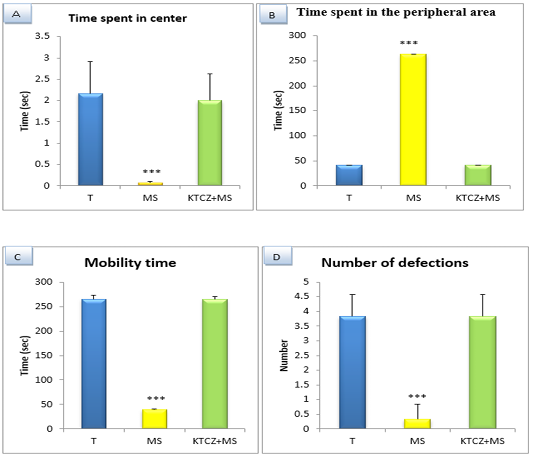
Figure 4: Changes in the parameters of the OF test following controls , maternal separation and maternal separation treated groups A. Time spent in center B. Time spent in the peripheral area (sec), C. Mobility time(sec) D. Number of defecation (T: Temoin, MS: maternal separation, KTCZ: ketoconazole, * p < 0.05;**p < 0.01; ***p < 0.001).
Anxiety-like behavioral development: The statistical analysis of results (Figure 3) revealed a significant decrease of the time spent in the open arms of the dispositive in stressed maternal separated rats compared to the control rats and consequently a very significant increase of the time spent in the closed arms in the stressed rats compared to the control rats.
Locomotion-like behavioral development: The results (Figure 4) Show a significant decrease of the distance travelled and the number of a turnaround in MS animals compared to control group and significant increase of the immobility time and a number of defecation.
DISCUSSION
Current evidence suggests that animals subjected to early life maternal separation from PND 1–7 for a duration of 90min have increased behavioral disorders and altered biochemical parameters during adult life.
Our results showed, that maternal separated rats displayed a significant increase in anxiety-like behavior compared to control group in EPM test, they spent relatively less time in the open arms and more time in the closed arms of the EPM, its due to the overactivation of the sympathetic axis which stimulates the immune system which increases the pro-inflammatory cytokines cerebral which in turn react on these neuronal receptors and alter the metabolism of tryptophan which induce the decrease of serotonin level. Neurotransmitter systems appear vulnerable to adversity early in life. Reduced serotonin receptor expression has been demon-strated in the prefrontal cortex, hippocampus (Ohta K-i Miki et al., 2014; Li et al., 2013) and raphe nucleus (Own et al., 2013; Jahng et al., 2010) following early stress in animal models, indicating that adversity impairs serotonergic signalling. Reports also indicate that early stress influences dopaminergic signalling; decreased activity of the mesolimbic dopaminergic system has been demonstrated following separation, once again in line with depression-like behavior (Jahng et al., 2010). Alterations in social (Franklin et al., 2011) anxiety (Li et al., 2011) and depression-like (Leussis et al., 2012) behaviours have each been associated with altered monoaminergic neurotransmission in several works. Similarly decreased expression of parvalbumin, a GABAergic.
It is well established that MS causes lasting negative effects on the brain and behavior (Smith et al., 1997), and it is widely used to model anxiety-like behaviors (Aisa et al., 2007; Ishikawa et al., 2015; Cao et al., 2016; Amini-Khoei et al., 2019; Sang-Seo et al., 2019), especially if we consider the frequency of comorbidity of these mood disorders. Other studies apply the MS daily, between postnatal days 2–14, for 3 h (Diehl et al., 2012; Amiri et al., 2016), especially those that correlate MS to mood disorders, since it is a critical period for the maturation of the hypothalamic-hypophysis-adrenal axis in rodents.
In the open field test, the animals submitted to MS showed a reduction in the traveled distance and an increase of immobility time wich represents a decrease in the exploratory activity. This avoidance of open areas is indicative of anxiety-like behavior, which was also evidenced in our EPM experiment, where the MS animals explored the open arms of the apparatus for a shorter time. In agreement, previous studies in this experimental model have demonstrated a reduced activity in the central area of the open field and in the open arms of the elevated plus maze (Ishikawa et al., 2015; Cao et al., 2016).
During the development of rats, olfaction is essential for the recognition of the mother and the udders (Blass et al., 1980) is after essential to the nest (Brown, 1982) and apparent or familiar partners (Kristensen et al., 2001).
The rat is an animal called macros mate because it has very good capabilities of detection of volatile molecules. The olfactory system is a sensory system specialized in the detection and processing of volatile chemical in-formation, the odorant molecules. Unlike vision, it is a relatively plastic sensory system. An unknown molecule can still be detected, even if the olfactory sensation is weak at the beginning, the olfactory system will develop later receivers to detect it more effectively (Duchamp-Viret, 2012). Sensory signals play a key role in establishing and main-taining the two-way relationship between mother and off-spring. From a young’s point of view, olfaction is essential for the recognition of the mother and the udders (Blass and Teicher, 1980), nest (Brown, 1982), related or familiar partners (Kristensen et al., 2001).
In on our results, MS animals showed a decrease in olfactory sensitivity compared to control, this decrease is directly related to the absence of nasal breathing that is to say a respiratory distress in altering air conditioning inspires (Kimnelman, 1993). The early nasal abstraction has an impact on the olfactory abilities that disturbed the orientation towards the nest. Stress, bilateral nasal obstruction could therefore be a stressful multi-factorial situation. In the case where it would actually result in the establishment of a stress response, the nasal obstruction would represent a chronic stressful situation, ie a relatively moderate disturbance lasting for several days (Pacak and polkovits, 2001).
Our result showed a remarkable increase in blood glucose level in MS animals to controls. According to (Hers, 1990; Nordlie et al., 1999; Abdollahi et al., 2003a,b). The liver plays a major role in blood glucose homeostasis by maintaining a balance between glucose uptake and storage via glycogenesis and glucose release through glycogenolysis and gluconeogenesis. In recent years the mechanisms affecting glucose homeostasis have been researched. Stimulation of gluconeogenesis and hepatic glycogenolysis is proposed as a mechanism responsible for OP-induced increase in blood glucose level (Abdollahi et al., 2004), the other mechanism is activation of the hypothalamic-pituitary-adrenal axis (HHS), activation of the HHS axis by OPs leads to the secretion of glucocorticoids and thus a possible increase in blood glucose (Rahimi and Abdollahi, 2007).
During stress, activation of the corticotropic axis begins with stimulation of the amygdala. This stimulation leads to the release of corticotropin-releasing hormone (CRH) in the pituitary portal system. CRH is the main regulator of the adenohypophysis adrenocorticotropic hormone (ACTH) secretion (Chrousos and Gold, 1992). In response to ACTH, the steroidogenic cells of the adrenal glands use cholesterol to produce steroid hormones. Among these, glucocorticoids (cortisol and corticosterone) are the ultimate effectors of the neuroendocrine response to stress (Ottaviani et al., 1996). Indeed, when an animal is facing a stressor, plasma cortisol levels (in most mammals) and corticosterone (in rodents especially) increase more or less rapidly depending on the nature of the intensity and duration of the stresseur. Activation of glucocorticoids are mainly related to carbohydrate, lipid and protein metabolism. The secretion of glucocorticoids leads to lysis of energy reserves and the increase of blood glucose level (Ottaviani et al., 1996). It is, in fact, global redistribution of energy in a way that facilitates the adaptation of the body (Bodey et al., 1999).
After the treatment by the ketoconazole, we noticed that there is not a significant difference between the rats separated for 90 min treated and control rats so in this treatment also ketoconazole highlighted the setting in the play of the axis corticotrope in the definitions of behavior. These relays engaged via the hypothalamic-pituitary-adrenal (HPA) axis seem to induce a change of behavior pace to a stressful situation.
It has been reported that Stress causes alteration in hematological parameters like increase in total and differential WBC counts, neutrophils (Takano, 1983; Lakshmi and Sudhakar, 2009). In our results, maternal separation stress increase wihte blood cells and monocytes counts and decrease neutrophiles, eiosinopillis and lymphocytes counts compared to controls. However, glucocorticoids may exert immunostimulatory properties of B cells play sometimes a role of immunostimulant and sometimes immunosuppressive (Steele, 2002). Leukocytes possess receptors for adrenaline, sex steroids, insulin, prolactin, growth hormone and thyroxine. Monocytes from bone marrow have not been only a phagocytic activity but also secrete enzymes , proteins , prostaglandins , cytokines and even ROS (Reactive oxygen species) and NO (reactive nitrogen species) (Pereira et al., 1996). Which are important in the defense against pathogens and tumor cells. (Hibbs et al., 1988; Macmicking et al., 1997). High levels of cortisol stimulate apoptosis of thymocytes and can cause lymphopenia and monocytopenia (Nascimento et al., 2004).
CONCLUSION
The study, therefore, concluded that ketoconazole has a potential antistress activity in maternal separation stress in rat. it prevented the stress-induced alteration in hematological, biochemical and behavioral parameters.
ACKNOWLEDGEMENT
The authors of this article would like to thank Dr. Toul Fethi for their scientific comments and for linguistic assistance.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHORS CONTRIBUTION
All authors contributed equally.
REFERENCES





