Advances in Animal and Veterinary Sciences
Research Article
Responses of the Four Larval Stages (L1 To L4) of the Avian Malaria Vector Culiseta longiareolata Exposed to Spinosad (Naturally Derived Insecticide)
Merabti Brahim1,2*, Molinari Manel1, Merah Imen1, Ouakid Mohamed Laid3
1Department of Biology, Faculty of Sciences, Amar Telidji University, B.P. 37, Laghouat, Algeria; 2Desertification and Climate Team, Mechanics Laboratory, Amar Telidji University, B.P. 37, Laghouat, Algeria; 3Department of Biology, Faculty of Sciences, Badji Mokhtar University, B.P. 12, Annaba, Algeria.
Abstract | The sensitivity of the first to fourth-stage larvae of Culiseta longiareolata (Diptera: Culicidae) treated with Spinosad was the first aim of this work. Five concentrations were administered for the toxicological tests (0.05, 0.1, 0.5, 1 and 5 μg/l). The results gained showed us an insecticidal effect which increases, according to the concentration and the time of exposure. The average lethal concentration LC50 of the L1 stage was in the order of 2.46 μg/l and the LC90 was 3.28 μg/l. For the L2 stage, the LC50 calculated was a 2.88 μg/l and LC90 with a 3.63 μg/l. For the L3 stage, the LC50 obtained was a 3.06 μg/l and the LC90 with a value of 3.88 μg/l. The LC50 of L4 stage calculated was a 3.16 μg/l, and the LC90 was a 3.93 μg/l. Based on the results of thisstudy, it was noted that the premature stages (L1 and L2) were sensitive to the product used as compared withthe tardy stages (L3 and L4) on all which was resistant according to the calculated toxicological parameters.
Keywords | Culiseta longiareolata, Culicidae, Spinosad,Toxicological tests, Toxicological parameters.
Received | January 24, 2019; Accepted | May 14, 2019; Published | May 30, 2019
*Correspondence | Merabti Brahim, Department of Biology, Faculty of Sciences, Amar Telidji University, B.P. 37, Laghouat, Algeria; Email: ecobiskra@hotmail.fr
Citation | Brahim M, Manel M, Imen M, Laid OM (2019). Responses of the four larval stages (l1 to l4) of the avian malaria vector culiseta longiareolata exposed to spinosad (naturally derived insecticide). Adv. Anim. Vet. Sci. 7(7): 599-603.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.7.599.603
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Brahim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Avian malaria and various arboviruses are very interesting diseases on the veterinary and ecological side, of which Culiseta longiareolata is the vector of these diseases (Maslov, 1989). It may also involve the transmission of causative agent of Malta fever (Maslov, 1989). It is also an avian plasmodium vector (Severini et al., 2009; Mughini-Gras et al., 2014). Cs.longiareolata is multivoltine with continuous development in hot countries with the a large distribution is present in the south of the Palearctic region; it is distributed in the Mediterranean region, Europe and Asia (Minar, 1991; AI-Khalili, 1997; Lee et al., 2009). Actually, there is a contradiction about many authors mentioned that this species attack human and animal, but the birds are only the principal host in North Africa (Maslov, 1989; Roubaud and Colas-Belcour, 1933).
In recent years, Cs.longiareolata has precisely become a very common species in the Algerian Sahara (Bebba and berchi, 2004; Merabti and Ouakid, 2011; Mengri et al., 1984; Merabti, 2016; Merabti et al., 2016; Merabti et al., 2017). In addition, the arrival of this species to the urban agglomerations, lead us to study it. Actually, the fight against transmissible disease vectors becomes very encouraged in the biology, ecology and public health areas. The researchers of new strategies and fight mean take a major magnitude. For this, the knowledge of same species vectors bioecology is necessary to achieve those goals. Knowing the most susceptible larval stage as the most resistant of Cs.longiareolata so the target stage to fight, and the effect of Spinosad on these four stages were the two focused aims of the present study.
MATERIALS AND METHODS
Larval Sampling and Breeding
Cs.longiareolata larvae were collected from different cottages in the region of Laghouat (33°48′24″N, 2°52′56″E). The larvae were reared in storage jars containing 500 ml of stored tap water and maintained at a temperature of 25–30°C, 85% RH and a photo period of 14: 10 (L: D). Larvae were fed daily with fresh food consisting of a mixture of biscuit-dried yeast (75:25 by weight) and water was changed every three days. The feeding continued until the larvae develop into pupae. The pupae were transferred from the trays to a cup containing tap water and placed in screened cages (30×30×30 cm) where the adults emerged. After emergence, female mosquitoes obtained a blood meal from caged pigeons, while male mosquitoes were fed a 10% sucrose solution. Egg masses were kept to continue the next generation.
Bioassays and Larval Mortality
The evaluation of the mortality rate of the mosquito population has been studied according to the recommendation of the World health organization (Rivero, 2010). Our bioassay was performed with L1, L2, L3 and L4 stages of Cs.longiareolata using five concentrations (0.05, 0.1, 0.5, 1 and 5μg/l) of Spinosad; composed of a mixture of two metabolites (Spinosynes A and D) synthesized from the bacterium Saccharopolyspora spinosa, from the group of Actinomycetes (Darriet et al., 2005) .20 larvae were incubatedin a beaker containing 200 ml of distilled water. Larval mortality was checked after 24 to 120 hrs of incubation. Each treatment was performed in triplicates. In all assays, the mortality of larvae was recorded and calculated according toAbbot formula (Abbott, 1925).
Statistical Analysis
The method of Swaroop et al. (1996) permits the calculation of the confidence interval (95%) of the LC50, LC90 (μg/l) and regression equationswere applied according to Finney’s mathematical methods. The data was transformed and normalized according to the Bliss tables (Finney, 1971). Calculations and descriptive statistics, as well as the chi-2 test (95% confidence interval) was applied to analyze variation between different stages in different concentrations, were calculated using Statistix v8.0
RESULTS
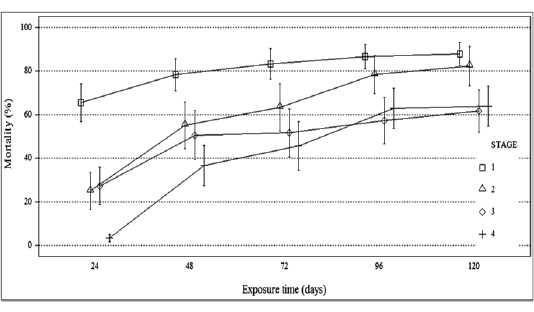
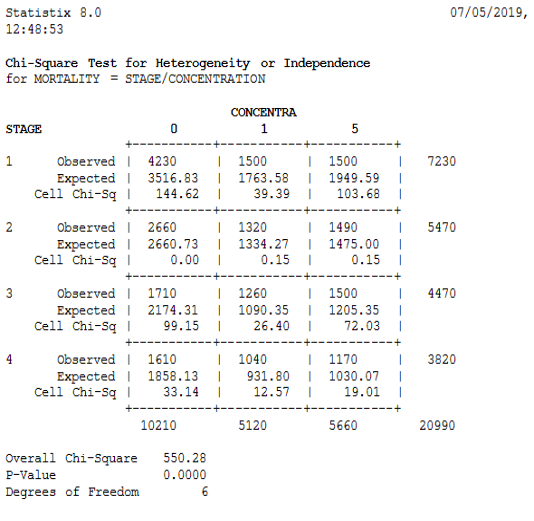
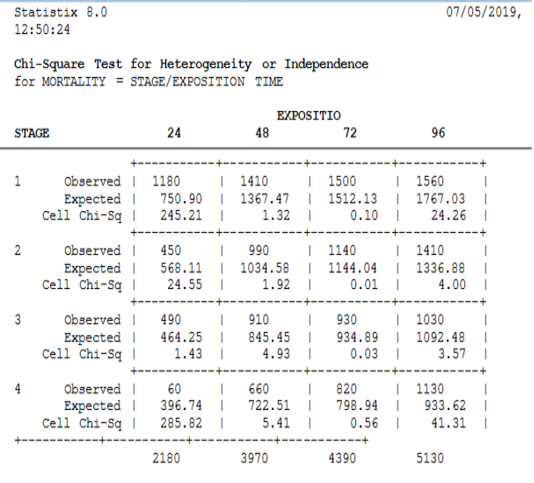
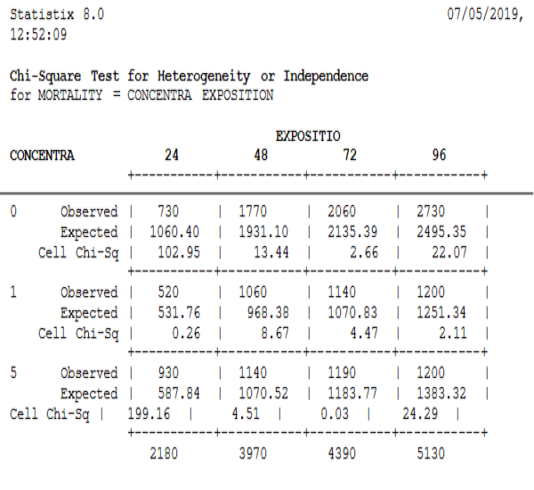
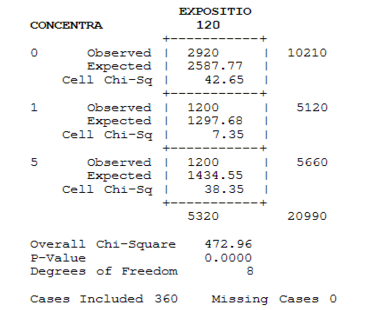
Figure 1 shows the responses of the four larval stages (L1, L2, L3, and L4) at the five concentrations used in the treatment with our product. Mortalities were recorded from 24h to 120h. According to this figure, the mortality rates recorded for the four stages were different (χ2=550.28, DF=6, P≤. 0001). For the first stage; the mortality appreared after 24 hours of treatment was greater than 65%.
While for the second and the third stages this rate was almost 30%. This rate was too low in fourth instar larvae (inferior to 5%). After this time of exposure, the mortality rate was a significant variable between the four stages (χ2=400.63, DF=6, P≤. 0001).
After 48 hours of treatment, the mortality rates recorded for the first stage are almost 80%, while the second and the third stages have a rate close to 50%. The mortality rate for the fourth stage was not more than 36%. So, the mortality again records a significant variation between those stages (χ2=155.72, DF=6, P≤. 0001).
For 72 hours of treatment, the rates observed for the first stage exceeded 80%, while the second, third and fourth stage have mortality rates ranging between 45% and 63%. There was a significant variation in the mortality rate among the fourth stages (χ2=168.26, DF=6, P≤. 0001).
p Larval stage 1.
r Larval stage 2.
t Larval stage 3.
+ Larval stage 4.
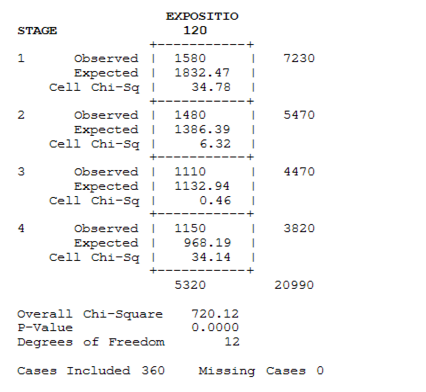
After 96 h to 120 h of treatment, the first two larval stages record high mortality which exceeded 90%, in addition, the third and fourth reacted with the concentrations used, whose rates did not exceed 63%. The variability was significant after the both exposure times, respectively (χ2=126.04, DF=6, P≤. 0001), (χ2=104.00, DF=6, P≤. 0001)
Table 1 presents the toxicological parameters calculated for the four treated larval stages. It has been noticed that the more the larva develops from one stage to another; it requires a high concentration for mortality to increase. Respectively with the four stages L1; L2; L3 and L4, the calculated LC50 were 2.05; 2.17; 3.03 and 3.53 μg/l. While the calculated LC 90 was respectively 3.39; 3.45; 3.74 and 3.78 μg/l for these stages.
Table 1: Lethal concentrations with confidence intervals for the mosquito species treated with Spinosad
Larvae stage |
LC50 μg/l |
LC90 μg/l |
LLC50 μg/l |
ULC 50μg/l |
LLC90 μg/l |
ULC 90 μg/l |
| L1 | 2,05 | 3,39 | 1,06 | 13,85 | 0,55 | 16,22 |
| L2 | 2,17 | 3,45 | 0,55 | 6,61 | 0,16 | 77,29 |
| L3 | 3,03 | 3,74 | 0,66 | 14,29 | 0,18 | 86,17 |
| L4 | 3,53 | 3,78 | 0,94 | 10,91 | 0,34 |
44,74 |
LC: lethal concentration, LLC: Lower Lethal Concentration, ULC: Upper Lethal Concentration.
DISCUSSION
The exposure of any animal population to a toxic substance may produce biochemical, histological or morphological effects resulting in specific alterations of an organ (Ilavazhahan and Selvi, 2012), these effects vary according to the intensity, way of action, frequency, and duration of exposure to this substance, but also according to the degree of sensitivity or resistance of the species itself (Sanchez-Bayo, 2012; Najar-Rodriguez et al., 2008).
Our results showed a good activity of spinosad like a larvicide on those immature stages. The results indicate mortality rates up to 100% for higher concentrations and at the precocious stages (L1 and L2). While for both L3 and L4, the responses were low compared to the first two stages.
Spinosad with its emulsifiable concentrate (EC) formula was a product that is being used more and more against several types of insects. The effects of this larvicide have been evaluated against several mosquito species. Several species have been used as a target for this product; for example Culex pipiens, Aedes aegypti, Anopheles gambia, Culex quinquefasciatus, Anopheles stephensis, Anopheles albimanus ... etc (Darriet et al., 2005; Romi et al., 2006; Bahgat et al., 2007).
According to O.M.S (2005), a recent study has shown that interim recommendations for the protection of marine life are based on an LC50% of 13 mg/l after 48h, for salt marsh mosquito larvae Aedes taeniorhynchus.
Further studies showed that the Spinosad effect results in variable mortality depending on the concentration used and the time of treatment for mosquitoes. After 15 days of treatment, the larval mortality rates increase and can reach 100% when using the highest concentrations (50 μg/l and 100 μg/l) and the LC50 is equivalent to 7.76 μg/l, while LC90 is equal to 44.67 μg/l (Bouzerida, 2016).
The work of (Darriet et al., 2005) shows the effect of Spinosad on sensitive strains of three mosquitoes of medical interest Ae. aegypti, An. gambiae and Cx. Quinque fasciatus showed that the LC50% are 0.35, 0.01 and 0.03 mg/lrespectively.
Another study done with a concentrate Spinosad (EC) Emulsifiable (EC) Assay 4.8% active substance showed an LC50% (0.0096 mg/l) on Ae aegypti; 0.0064 mg/l on Cx. pipiens and 0.039 mg/l on An.Stephens is (Romi et al., 2006). By comparing to the tests carried out with the active substance the greater larvicide efficacy of Spinosad in its (EC) form could be explained by the oily nature of the wording that would prevent Mosquito larvae breathe on the surface of the water. Field evaluations are still scarce on Ae. aegypti, a study showed that at the concentration of 10 mg/l, the efficacy of this larvicide was totally over a period of five months (Bond et al., 2004), therefore, its efficacy at 10-2 and 10-3 mg/l concentrations, mosquitoes have made him an excellent choice in vector control. According to elaborate treatments for different species of mosquitoes, we have noticed the degree of sensitivity of several species, through calculated LC 50 and CL 90.
Therefore, several studies that profoundly address this issue of resistance and sensitivity, which can be related to the height, age, or weight of the individuals subjected to toxicological tests. Ranson, H., & Lissenden (2016) work on the resistance mechanism on mosquitoes species and the combined effect of somes insecticides. Strode et al. (2006) worked on increasing resistance against some toxic substances as a function of age development in Anopheles gambiae.
CONCLUSION
The treatment with Spinosad for 24 h to 120 h of exposure induces a toxic effect on all four larval stages tested. Spinosad was used at different concentration (0.05; 0.1; 0.5; 1 and 5 μg/l), which determined the remarkable lethal concentration (LC50 and LC90), this effect was translated into a mortality rate that has reached an almost maximum rate of 100% for the more common concentration higher. The sensitivity of the L1 and L2 stages has been well noticed compared to L3 and L4, for this reason, the treatment of the more resistant stage than to the approval is recommended.
CONFLICT OF INTEREST
The authors have notany conflict of interests.
ACKNOWLEDGMENTS
The study was supported by Laghouat University. We are obliged to Dr. A-E. Adamo for remarks and consultation.
authors contribution
Merabti Brahim: Methodology, protocols, statistical and toxicological analysis and drafting of documents.
Molinari M and Merah I: Mosquito sampling, breeding and toxicological treatment.
Ouakid M L: Correction and general revision of document.
REFERENCES