Advances in Animal and Veterinary Sciences
Research Article
Comparison of Microbiological Quality Between Organic and Conventional Goat Milk: A Study Case in Bogor, Indonesia
Veronica Wanniatie1,2, Mirnawati B. Sudarwanto3*, Trioso Purnawarman3, Anuraga Jayanegara4
1Graduate School of Veterinary Public Health, Bogor Agricultural University, Indonesia; 2Department of Animal Husbandry, Faculty of Agriculture, University of Lampung, Indonesia; 3Departement of Animal Diseases and Veterinary Public Health, Faculty of Veterinary Medicine, Bogor Agricultural University, Indonesia; 4Department of Nutrition and Feed Technology, Faculty of Animal Science, Bogor Agricultural University, Indonesia.
Abstract | The aim of this study was to compare microbiological quality between organic and conventional goat milk in Bogor District, West Java Province, Indonesia.Between March to August 2018, a total of 36 bulk tank milk samples were collected from 3 locations of organic and conventional goat farms. The milk samples were determined for bacteria population (total plate count, Staphylococcus aureus, Enterobacteriaceae and coliform) and parasite population (Toxoplasma gondii, Entamoeba sp.and Balantidium sp.). The determination was according to US FDA-BAM (United States Food and Drug Administration–Bacteriological Analytical Manual) for Aerobic Plate Count analysis. Data were analyzed by Mann Whitney test. The presence of parasite was analyzed descriptively. Results showed that the populations of total bacteria, S. aureus, Enterobacteriaceae and coliform present inorganic goat milk were 5.58, 3.51, 4.32 and 3.69 log cfu/ml, respectively. On the otherhand, total bacteria, S. aureus, Enterobacteriaceae and coliform populations present in conventional goat milk were 5.02, 2.89, 4.12, and 2.46 log cfu/ml, respectively. Population of S. aureus, Enterobacteriaceae, and coliform in organic goat milk exceeded the maximum limit of the Indonesian National Standard, whereas for conventional goat milk only S. aureus and Enterobacteriaceae exceeded the maximum limit. T. gondii was not found either in organic or conventional goat milk, but other types of protozoa, i.e. Entamoeba sp. and Balantidium sp. were found. In conclusion, microbiological quality of organic and conventional goatmilk in Bogor was relatively similar and appropriate within the Indonesian National Standard.
Keywords | Organic milk, Goat, Bacteria, Protozoa, Microbiology
Received | December 31, 2018; Accepted | March 30, 2019; Published | May 28, 2019
*Correspondence | Mirnawati B Sudarwanto, Departement of Animal Diseases and Veterinary Public Health, Faculty of Veterinary Medicine, Bogor Agricultural University, Indonesia; Email: mwanto47@hotmail.com
Citation | Wanniatie V, Sudarwanto MB, Purnawarman T, Jayanegara A (2019). Comparison of microbiological quality between organic and conventional goat milk: a study case in bogor, Indonesia. Adv. Anim. Vet. Sci. 7(7): 593-598.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.7.593.598
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Wanniatie et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Organic milk is defined as milk that free of hazardous materials, originating from livestock based on environmental sustainability and animal welfare. It is produced in organic farms that apply minimum standards to ensure livestock well-being (Sundrum, 2001; Schwendel et al., 2015). Presently the demand for organic milk is increasing (Liu et al., 2013; Malissiova et al., 2015) since it is believed to promote health better as compared to conventional milk (Malissiova et al., 2015). Organic milk products are free from antibiotics, hormones, synthetic chemicals, and genetic modification; and they should be beneficial for human health (Schwendel et al., 2015). Despite its benefit, risk of parasitic contamination in dairy products from organic farms is higher than those from conventional farms (Sundrum, 2001) due to the typical grazing system. In a previous study, organic milk from cows was contaminated with a number of microbes such as coliform bacteria, S. aureus, Enterobacter aerogenes and Proteus vulgaris (Iqbal et al., 2016). In addition, protozoa species such as Toxoplasma gondii as also found in raw goatmilk, and it had been studied in the United States, Brazil, Greeceand Indonesia (Dubey et al., 2014; Da Silva et al., 2015; Sadek et al., 2015; Saridewi et al., 2015). T. gondii can cause toxoplasmosis, an intracellular anobligate cosmopolitan zoonosis, when infects humans and warm-blooded animals. Prevention can be done to avoid contamination of T. gondii in goat’s milk by improving sanitation management. T. gondii in goat’s milk can be transmitted to humans if it was raw consumed or used without pasteurization (Dubey et al., 2014; Da Silva et al., 2015).
In Indonesia, dairy goats have not received much attention as compared to dairy cows (Taufik et al., 2011). Meanwhile, the tradition in drinking raw goat milk is still common for many consumers, which is believed that it can enhance health (Claeys et al., 2013) beside better taste and nutrition (Taufik et al., 2011). However, raw milk might contain microbial pathogens such as S. aureus, Escherichia coli, Listeria, Campylobacter and Salmonella (Hill et al., 2012; Kalmus et al., 2015), and zoonotic pathogens such as T. gondii (Dehkordi et al., 2013). To date, comparison of microbiological quality (including the pathogens) between organic and conventional goat milk has never been previously investigated in Indonesia. Therefore the aim of this research was to compare the microbiological quality, i.e.total plate count (TPC), S. aureus, Enterobacteriaceae, coliform and parasites between organic and conventional goat milk in Bogor District, West Java, Indonesia.
Materials and Methods
Study Area and Milk Sampling
Thirty six individual milk samples were collected from six dairy goat farms all over Bogor District, Indonesia, in March to August 2018. Milk samples were collected from two types of goat farms, i.e., organic and conventional goat farms. We used a composite samples from Etawa and Sapera goats. Samples were collected once a month for 6 months. Every 500 mL of bulk was collected in sterile glass bottles and transferred to laboratory under cool condition (5°C). All samples were analyzed immediately at Protozoology Laboratory and Veterinary Public Health, Faculty of Veterinary Medicine, Bogor Agricultural University.
Procedures
The tests carried out in this research were according to Kornacki dan Johnson (2001). This research consisted of some methods like conventional culture of TPC, whereas for the total S. aureus count, Enterobacteriaceae count, and coliforms count. The number of bacteria was calculated with count method by pouring. Some agars had been used in this study such as Plate Count Agar (PCA) (CM325 Oxoid), Vogel Johnson Agar (VJA) (CM641 Oxoid), MacConkey Agar (MCA) and Violet Red Bile Agar (VRBA) (CM485 Oxoid) (AOAC 2005). The presence of parasites was done by detection of tachyzoites and trophozoites. The method used microscopic test with Giemsa and Lugol stain. To observe thetachyzoites and trophozoites a preparate of samples examined thoroughly with a microscope using an oil immersion objective lens (100×) (Sadek et al., 2015; Al-Habsi et al., 2017).
Statistical Analysis
The data obtained were analyzed by Mann Whitney test. Kolmogorov-Smirnov and Shapiro Wilk tests were applied to perceive normality data. The presence of parasite was analyzed descriptively.
Results
There was no difference in bacteria population (TPC,S. aureus, Enterobacteriaceae and coliform) between organic and conventional goat milk (Table 1). The total bacteria population in both organic and conventional goat milk was still below the Indonesian National Standard threshold and EU Council Directive 92/46/EEC. However, Enterobacteriaceae count in both organic and conventional goat milk was above the SNI threshold (3.00 log cfu/ml), i.e. 4.32 and 4.12 logcfu/ml, respectively. The number of S. aureus and coliform in organic goat milk were higher than conventional goat milk namely 3.51 and 3.69 log cfu/ml, respectively, and they exceed both SNI and EEC maximum standard.The TPC and Enterobacteriaceae were positively correlated with coliform count in organic goat milk (P<0.05) (Table 2). Similarly, the count of TPC in conventional goat milk showed a positive correlation with coliform count (Table 3). T. gondii was not detected, however, Entamoeba sp. and Balantidium sp. were found in both organic goat milk and conventional goat milk (Table 4; Figure 1).
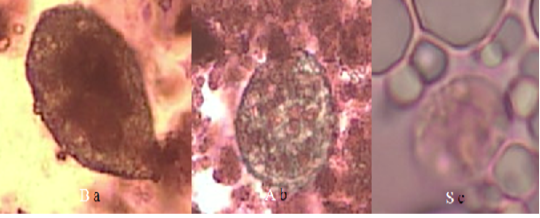
Figure 1: Form of protozoan trophozoites in goat’s milk with Lugol’s staining (40x magnification) (a) Balantidium sp. in organic goat milk; (b) Balantidium sp. in conventional goat milk; (c) Entamoeba sp. in conventional goat milk.
Discussion
The S. aureus grows on VJA was showed colored black colonies, convex, shiny surrounded by yellow zones (Baird and Lee, 1995). Enterobacteriaceae have been grown on MCA shows two different types of colonies such as red or pink
Table 1: Bacteria population (log cfu/ml) presentin organic and conventional goat milk compared to the standard maximum limit
| Bacteria | Organic (mean±SD) | Conventional (mean±SD) | P value | Indonesian National Standard for Fresh Milk | EU Council Directive 92/46/EEC (raw goat and sheep milk) (EC, 1992) | |
| Total plate count | 5.58±1.25 | 5.02±0.91 | 0.137 | 6.00 | 5.70 | |
| S. aureus | 3.51±1.32 | 2.89±1.56 | 0.206 | 2.00 | 3.30 | |
| Enterobacteriaceae | 4.32±1.51 | 4.12±1.56 | 0.670 | 3.00 | - | |
| Coliforms | 3.69±2.05 | 2.46±1.86 | 0.067 | 3.00 | 2.00 | |
Table 2: Correlation matrix of microbiological quality in organic goat milk (n =18)
| Parameter | TPC | S. aureus | Enterobacteriaceae | Coliforms |
| TPC | 1.000 | 0.381 | 0.441 | 0.589* |
| S. aureus | 1.000 | 0.011 | 0.327 | |
| Enterobacteriaceae | 1.000 | 0.494* | ||
| Coliform | 1.000 |
Table 3: Correlation matrix of microbiological quality in conventional goat milk (n = 18)
| Parameter | TPC |
S. aureus |
Enterobacteriaceae | Coliforms |
| TPC | 1.000 | 0.302 | 0.423 | 0.709** |
| S. aureus | 1.000 | 0.312 | 0.310 | |
| Enterobacteriaceae | 1.000 | 0.393 | ||
| Coliform | 1.000 |
Table 4: Presence of T. gondii, Entamoeba sp. and Balantidium sp.(in percentage) in organic and conventional goat milk
| Protozoa | Organic (n = 18) | Conventional (n=18) |
| T. gondii | 0 | 0 |
|
Entamoeba sp. |
0 | 16.7% |
|
Balantidium sp. |
33.3 % | 11.1% |
(bacteria that ferment lactose like Escherichia coli and Klebsiella sp.) and colorless or transparent (bacteria that do not ferment lactose such as Salmonella sp. and Shigella sp.) (Kornacki dan Johnson, 2001). The coliform grows on VRBA was showed all purplish red colonies, surrounded by red zones (Kornacki dan Johnson, 2001).
The presence of microbes in this study was seen from TPC, S. aureus, Enterobacteriaceae, and coliform in organic and conventional goat milk with no difference. This result similar to another study conducted by (Sundrum, 2001), which was found no difference in microbial count between organic and conventional goat milk. TPC is the best way to assess milk management and quality by calculating bacterial density in milk and estimating the amount of milliliter aerobic bacteria. TPC values can be high if contamination occurs from milking equipment, dirty udders, and livestock are suffered to subclinical or clinical mastitis (Cicconi-Hogan et al., 2013). In this study we presumed that contamination occur from milk milking since it still used manual milking rather than machine milking.
There was no significant difference between organic and conventional goat milk in S. aureus count. Although higher in organic udder, the presence of S. aureus in bulk tank milk was associated with mastitis, age of the cage and a higher percentage of cows with 3 or fewer teats in both the organic and total herd (Cicconi-Hogan et al., 2013). S. aureus can originate from mastitis which can be contagious causes IMI, furthermore it will the affects milk production and somatic cell count (SCC) (Keefe, 2012). S. aureus can spread easily from animal to animal through a milking hand or milking device.
The total of coliform in organic goat milk was higher than conventional, although it was not significantly different. In contrast, research in the United States (Iqbal et al., 2016), the total coliform in organic cow milk was lower than conventional cow milk. The presence of coliforms, such as E. coli and Klebsiella spp., in milk indicates contamination, that can occur from dirty environments and equipment (Iqbal et al., 2016) and can come from mastitis infected livestock (Cicconi-Hogan et al., 2013). Besides coliform, another bacteria also contaminate organic cow milk in Pakistan, especially Staphylococcus aureus, and Proteus vulgaris.
The count of TPC and Enterobacteriaceae had a correlation with the amount of coliform in organic goat milk (P <0.05). This result shows that the high of coliform increases the number of total plate count and Enterobacteriaceae. Coliform bacteria are used as microbial indicators of cleanliness in the dairy industry. In addition to coliform was also Enterobacteriaceae used as an indicator of cleanliness in the dairy industry (Hervert et al., 2016). Coliform bacteria consist of Escherichia, Klebsiella, Citrobacter, and Enterobacter, although more than 20 gram negative genera meet the phenotypic coliform criteria (Masiello et al., 2016). The Enterobacteriaceae family includes most coliform bacteria usually lack the ability to ferment lactose such as Salmonella and Yersinia (Imhoff, 2005).
In this study there was no T. gondii found in both milk samples (Table 3). T. gondii was an intracellular protozoa which cause of toxoplasmosis infect attack warm-blooded animals and humans. Tachyzoites T. gondii is found in the milk of sheep, goats, cows, and rats (Dubey et al., 2014; Da Silva et al., 2015; Sadek et al., 2015). Infection in humans was transmitted through drinking consumption of raw goat milk practice (Skinner et al., 1990) and suspected transmission through lactation can also occur in humans (Bonametti et al., 1997). Toxoplasmosis can occur through drinking unpasteurized goat’s milk (Skinner et al., 1990; Saridewi et al., 2015). Excretion of tachyzoites in milk comes from naturally infected goats with T. gondii. Although infections occur due to drinking goat’s milk, other researchers report that drinking raw milk products from other animals can also cause transmission of T. gondii horizontally (Kijlstra & Jongert, 2008; Dubey et al., 2014).
Although T. gondii was not found, we have found another gastro intestinal parasites namely Entamoeba sp and Balantidium sp. The risk of parasitic contamination in dairy products from organic farms was higher. It’s due to more intensive grazing system in organic farm than conventional farm because organic farms use a more intensive grazing system compared to conventional farms (Sundrum, 2001). Gastrointestinal parasite was an unusual problem in small ruminant production systems (Rahman et al., 2017). Gastrointestinal parasites such as worms and protozoa are very common in goats and sheep [29] so it becomes an infection and can reduce milk and meat production (Murthy & Rao, 2014). Diseases caused by gastrointestinal parasites can interfere with health and harm the economy of farmers (Asif et al., 2008).
Entamoeba sp. was found in conventional goat milk as much as 16.67% (3 samples). Gastrointestinal parasites such as Entamoeba sp. can be found in goat’s milk probably because of contamination from animal or human feces. Entamoeba sp. has been found in goats in Kenya (Kanyari et al., 2009), Thailand (Sangvaranond et al., 2010), Cameroon (Ntonifor et al., 2013), Brazil (Radavelli et al., 2014), Nigeria (Adua et al., 2017) and Australia (Al-Habsi et al., 2017).
Balantidiumsp. was found in organic goat milk (33.33%) and conventional goat milk (11.11%) in the Bogor District. Balantidium sp. cysts and trophozoites found 7.1% (n = 14/198) in goat intestines and stools in the Egyptian region (Elmadawy & Diab, 2017). The prevalence of Balantidium coli in Tanzania was 4.8% (Mhoma et al., 2011) whereas in Kenya, the prevalence was 3%, which is mostly a mixed infection with other parasites (Kanyari et al., 2009). Goat milk has the opportunity to be contaminated with Balantidium sp. during the milking process. Cyst B. coli that contaminate food and water can come from human and animal feces. Balantidiasis in humans occurs due to contact with sheep, goats and pigs (Yazar et al., 2004; Jamil et al., 2015).
Conclusion
The results showed no differences in TPC, S. aureus, Enterobacteriaceae, and coliform between organic and conventional goat milk. The amount of S. aureus, and coliform in organic goat milk exceed the Indonesian National Standard. This is due to the possibility of organic maintenance practices allowing for high amounts of microbes in livestock and in the enclosure environment. T. gondii is not found in organic and conventional goat milk, but another parasite was found, namely Entamoeba sp.trophozoites in conventional goat milk and Balantidium sp. in organic and conventional goat milk. The presence of Entamoeba sp. and Balantidium sp. in milk is probably due to fecal contamination.
Acknowledgments
This research was supported by BUDI-DN (Beasiswa Unggulan Dosen Indonesia-Dalam Negeri) scholarship from Indonesian Ministry of Research, Technology and Higher Education.
Conflict of Interest
All authors declare that there is no conflict of interest.
Authors contributions
VW performed sample collection, data analysis and wrote the manuscript draft. MBW, TP and AJ designed the study, supervised the experiment and revised the manuscript. All the authors have read and approved the final manuscript
REFERENCES





