Advances in Animal and Veterinary Sciences
Research Article
Effects of in- vivo Application of Cold Atmospheric Plasma on the Leukocyte of Balb/c Mice
Ansam Q Gadhban1*, Hamid H Murbat1, Khalid J Khalil2
1University of Baghdad, College of Science for Women, Department of Physics, Iraq; 2University of Al-Mustansiriyah, Iraqi Center for Cancer and Medical Genetic Research, Iraq.
Abstract | The current study included the direct effect of CAP on a number of leukocytes in the laboratory mouse by exposing the skin to CAP using the floating electrode dielectric barrier discharge (FE-DBD).The study was conducted on mice which were (2-3) months of age with the average weight of 38g. 250 mice were divided into two groups. Group I is exposed to a single dose of CAP and group II is exposed to multidose of CAP. Every group was divided depending on the exposure time and time after exposure into 5 subgroups. Exposure process has by using Floating Electrode -Dielectric Barrier Discharge for (15,30,60,120) sec and a sub-group is left as a control group. Blood samples are taken after (1, 2, 3, 7, and 14) days from exposure. Leukocytes were measured by using count blood cells device. The results revealed that CAP causes a significant decrease in number of leuckocyte after1day from exposure, then recovery was proportional with a dose of CAP and with sampling time as well. This may indicate stimulation of bonemarrow to produce more leuckocyte and eventually indicates that CAP effect was transported far from the site of exposure.
Keywords | Cold atmospheric plasma, Floating electrode -dielectric barrier discharge, Leukocytes.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 21, 2018; Accepted | November 24, 2018; Published | January 18, 2019
*Correspondence | Ansam Q Gadhban, University of Baghdad, College of Science for Women, Department of Physics, Iraq; Email: ansamqassim087@gmail.com
Citation | Gadhban AQ, Murbat HH, Khalil KJ (2019). Effects of in- vivo application of cold atmospheric plasma on the leukocyte of balb/c mice. Adv. Anim. Vet. Sci. 7(4): 250-255.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.4.250.255
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Gadhban et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Plasmais the fourth case of essential matter in the universe, with the other states familiar: solid, liquid, and gas (Li et al., 2017; Pandit et al., 2017). the overall plasma charge quasi-neutrality according to its temperature eplasma can be classified into two types, thermal and nonthermal, which are based on the temperature of the electrons compared to the other species (Kroesen et al., 2009; Graves 2012; Barekzi and Laroussi, 2013; Guerrero-Preston et al., 2014) . Nonthermal plasma is also called cold atmospheric plasma (CAP) which defined an ionized gas generated at near room temperature, in which electrons and heavy particles are in thermal non-equilibrium (Keidar, 2015, Lu et al., 2016). Two main methods have been widely used to generate CAP namely direct and indirect discharges , based on this two types, two CAP devices, CAP jet (Shashurin et al., 2010; Georgescu and Lupu, 2010; Kim et al., 2010; Yan et al., 2012; Ja Kimg et al., 2013) and dielectric barrier discharge (8DBD) (Fridman et al., 2007; Vandamme et al., 2011; Kaushik et al., 2012; Arjunan et al., 2012) have been developed and widely used in plasmaa medicine.
Plasma medicine is now a rapidly advancing field with positive indications of plasma’s ability to sterilize many types of surfaces, (Dobrynin et al., 2007; Cooper et al., 2008; 2009) including human and animal tissues, treat wounds (Fridman et al., 2008; Dobrynin et al., 2009), coagulate blood, cancer treatment (Fridman et al., 2006; Kalghatgi et al., 2007) and even treat diseases, when dealing with such diseases, this requires knowledge the effect of the plasma on blood components. Yang et al. (2015) presented a significant apoptosis of different cancer cells with preservation of normal; cells from and killing effect of CAP.
Dayun Yan and his colleagues in 2016 (Yan, Sherman, and Keidar, 2017) proved that CAP effect initially occur within the culture media by creating reactive Oxygen species (ROS) and reactive Nitrogen species (RNS) and the effect is consequently transported inside the cells where these species interact with mitochondria and induce the programmed death (apoptosis).
Also, they found that if they expose the culture media to CAP then keep it in a refrigerator, the effect of CAP will remain within the media for several days up to a week and when they seeded cells in these media they showed the same response as if they directly expose them to CAP.
Several research (Dobrynin et al., 2011; Dobrynin et al., 2011; Abdullah et al., 2017; Abdullah et al., 2017) simulate skin byescafold to study the penetration depth of CAP and they noted that it can reach cells deep within the dermis about 4mm from the surface of skin.
Depending to all these data, we tried inthis work to exposing the skin of mouse to CAP and study the possibility of transportation of its effect through the interstitial fluids of epidermis and dermal layers and reach blood and study its effect on number of leukocyte. Any immune modulation can be detected, it may have some implications in Oncology and it can be proposed that it is possible to treat any deep organ in the body by exposing skin to CAP in certain dosages.
Materials and Methods
Design and Construction of FE-DBD
The FE-DBD was designed and manufactured in Physics Department ∕ College of Science for Women laboratory:
1-Probe: it constint of Pyrex glasstube with diameter 20mm and length 10cm. This0tube is filled by0NaCl solution to increase its conductivity. A stainless-Steelerod with diameter 2 mm and1length 4cm is inserted in the solution and fixed in the nozzle of the tube by insulator material and 2 mm of this rod were left outside the tube. The external part of rod was connected0by a cable of length 1m to the power supply.
2-Holder: It consists of a plastic tube of length 12 cm and inner diameter 25mm and covers the cable at the probe end. The probe was installed on one end of the plastic1tube so that the end of the cable passed through the0tube. The cable is connected to the highivoltage powerssupply.
When approaching the probe of the skin or living tissue at a distance of 3 mm or less, the collapse occurs and generates CAP between the probe and the sample (animal skin).
Animals’ Model
The laboratory mice (Bulb\c type) were purchased from National Center for Drug Control and Research/ Ministry of Health / Baghdad/Iraq. 250 mice (male)owithage (2-3) months and average weighted is 38ig were kept in cages with a metal clip and a place suitable for breeding in terms of ventilation, lighting and appropriate temperature.
Hair was removed in the dorsal side of the mice using commercially available hair remover (veet). Hair removal is to ensure direct contact, with the skin from the CAP.
Exposure to Cold Atmospheric Plasma
A total of 250 mice were divided into 2 groups, one group was exposed to single dose of CAP and the other group is exposed to multiple doses of CAP (single dose every 48h) (0,48h ,96h). Every group is divided into 5 subgroups each consists of (25) mice (one of subgroup is left as control group) and the other groups were exposed to CAP for differenteexposure times (15, 30,60,120) sec.
Mice are left in differentilabeled cages for (1,2,3,7,14) day then blood samples are taken to be examined by WBCs counting (mindray).
The Process of Blood Collection
Blood samples were collected from animals by withdrawing blood directly from the heart using medical syringe. Blood was put in sterile tubes that contain anticoagulant K3-EDTA.
Blood samples were examined in the Iraqi Center for Cancer Research and Medical Genetics by Mindray.
Statistical Analysis
Linear1and2nonlinear4statistical1analyzes were performed with Prism (GRAPHPAD). Data were expressed as meanndeviation Standard Deviation Comparisons between two groups were analyzed using a t-test, and comparisons between more than two groups are analyzed by multiple t test –one per row.
Results
Single Dose
The results of single dose of CAP on leukocytes were summarized in Table 1. It was found that the influence of CAP on the number of leukocyteis differs with doses (15,30,60,120) sec and the period time after exposure (1, 2, 3, 7 and014) day.The influence of CAP a function of sampling day for every exposure time (dose) is shown in the figure 1(a, b,c, and d). Figure 2 (a, b, c, d and e) the showed the influence of CAP as a function of exposure time (dose) for differentssample day.
It was found that leukocyte count is different with different dosage of different sampling day. In general, leukocyte count decreases with all doses immediatelyaafter exposure in comparison with control group as demonstrated in figure (1.a), and this decline is directly proportional to the dose. After 2 to 14 days the decline is inverselyoproportional to dosesashown in figure (1.b, c, d, and e). After 3 days leukocyte count becomes more than that of control group, it was found that the maximum value of leukocytes at exposure time 120 sec where the amount of increase was with percentage 16% in comparison with control group but the increases is not significant asishown in figure (1.c).
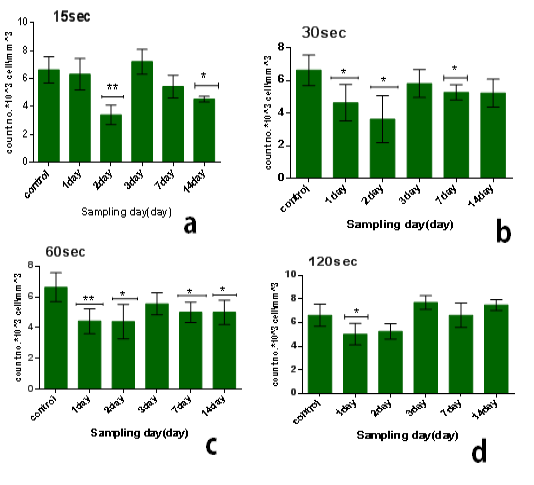
Figure 1: Effect of single dose of CAP on the count of leukocytes against with time measured after exposure (1,2,3,7,14) day,with stabilized dose of plasma (A): 15sec,(B): 30sec, (C): 60sec, (D): 120sec. Data are expressed as means+SD,*p<0.05 ,**p<0 .01, ***p<0 .001, (*) compared with control group.
Table 1 : Effect of single dose of cold plasma on leukocytes
| Exposure time | Count no.*103 cell\ml | ||||
| 1days | 2days | 3days | 7 days | 14 day | |
| 0 s | 6.6±0.9 | 6.6±0.9 | 6.6±0.9 | 6.6±0.9 | 6.6±0.9 |
| 15 s | 6.3±1.1 | 3.4±0.7 | 7.2±0.9 | 5.4±0.8 | 4.5±0.2 |
| 30 s | 4.6±1.1 | 3.6±1.5 | 5.8±0.9 | 5.3±0.5 | 5.2±0.9 |
| 60 s | 4.4±0.8 | 4.4±1.1 | 5.6±0.7 | 5±0.7 | 5±0.8 |
| 120 s | 5±0.9 | 5.3±0.6 | 7.7±0.6 | 6.6±1 |
7.5±0.5 |
On the other hand, the lowest-reading in the number of leukocyte was at the1time of exposure 15 sec and after 2 day from exposure, the amount3of the decrease compared to the control7group with percentage 48% and the difference was8significance (p<0.01), as shown in figure (2.b).
We1also found that the influence of one dose of CAP on the long term (after 14 days) from exposure showed a decrease in the no.of leukocyte at the low doses between 15-60 sec in comparison with control group with percentage almost 25% and with significant difference (p<0.05) but the no. Will increase for high doses 120 sec in comparison with control group with percentage 13% & significant difference (p<0.001) as shown in6figure (2.e).
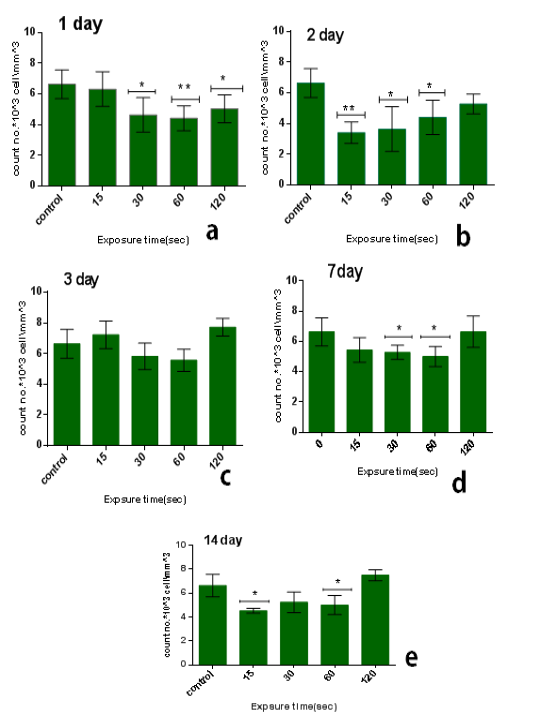
Figure 2: Effect of single dose of CAP on the count of leukocytes against with exposure time (15, 30, 60,120) sec with stabilized time measured after exposure (A): 1 day, (B): 2 day, (C): 3 day, (D): 7 day, (E): 14 day. Data are expressed as means+SD,*p<0.05 ,**p<0 .01,***p<0 .001, (*) compared with control
Multidose
The results of multi dose of CAP on leukocytes were summarized in Table 2, it was found that the influence of multi dose of CAP is behave nearly similar to that of single dose, but differed in two ways. The first is that decline continues beyond 7 days after which recovery started to take place. The second point is arise as the multidose effect is unstable or not constant relative to that of single dose.
From the results in the Table 2 we noticed that the no. of leukocyte2at the low doses (15) sec in the first days from exposure decreases in comparison with control group while the no. of leukocytes gradually increased whenever the more time after exposure as shown in figure (3.a). also when the dose increases at the time of exposure (30 and 60) sec the no. of leukocytes increase after first day but start to decreases when the interval after exposure increases (figure 3b & c), and at the time of exposure 120 sec the no. of leukocyte decreases after first day and start to increases slightly but stay less than control group when the interval after exposure increases as shown in figure (3.d). The lowest reading of the number of leukocytes was detected at the time of exposure 15 sec and after 2 day from exposure.The amount of decrease was compared to the control group with percentage 76% with significant differences between them (p<0.001) as shown in figure (4.b), but it was found the maximum value of leukocyte was at the time of exposure 15 sec and after 14 days from exposure with percentage 28% with significant differences between them (p<0.0001) as shown in figure (4.e).
Table 2: Effect of multidose of cold plasma on leukocytes
| Exposure time | Count no.*103cell\ml | |||||
| 1days | 2days | 3days | 7 days | 14 day | ||
| 0 s | 10.0±1 | 10.0±1 | 10.0±1 |
10.0±1 |
10.0±1 | |
| 15 s | 7.6±1.8 | 2.4±0.4 | 3.9±0.5 | 9.4±0.7 | 12.8±0.6 | |
| 30 s | 10.8±0.5 | 5.3±1.4 | 7.6±0.5 | 7.7±0.6 | 5.9±1.1 | |
| 60 s | 11.0±1.5 | 6.5±0.8 | 6.0±1.1 | 7.1±0.9 | 7.5±0.9 | |
| 120 s | 2.7±0.5 | 5.5±0.7 | 5.9±2 | 5.8±0.8 | 9.3±0.9 | |
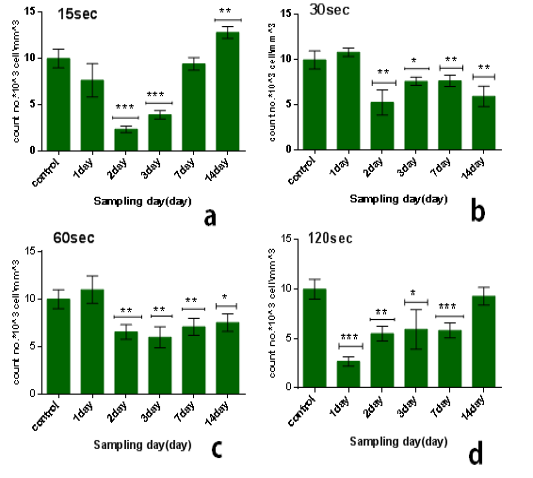
Figure 3: Effect of multi dose of CAP on the count of leukocytes against with time measured after exposure (1,2,3,7,14) day ,with stabilized dose of plasma (A): 15sec, (B): 30sec, (C) : 60sec, (D): 120sec. Data are expressed as means+SD,*p<0.05 ,**p<0 .01, ***p<0 .001, (*) compared with control group.
From another side, the influence of multidose8of CAP on the long term (after 14 days from exposure) at the low doses it was stimulationeeffect where the no. of leukocyte increases’ in comparison with control group-with percentage 28% significant different )(p<0.001) such as shown inifigure (3.d), but when the exposure timeeis doubled 30sec the no. of leukocytes decreases in comparison with control group with percentage 41% and significanttdifference (p<0.001) figure (3.b). But by increasing the exposure time, the number of leukocytes increases more than at 30 sec but remains below the control group ,where at the time of exposure 60 sec after 14 days the no. of leukocytes decreases in comparison with control group with percentage 25% and the significant difference (p<0.05), while at the time of exposure 120sec the no. of leukocytes decreases slightly incomparisonpwith control group with7percentage 7% and no significant difference between them.
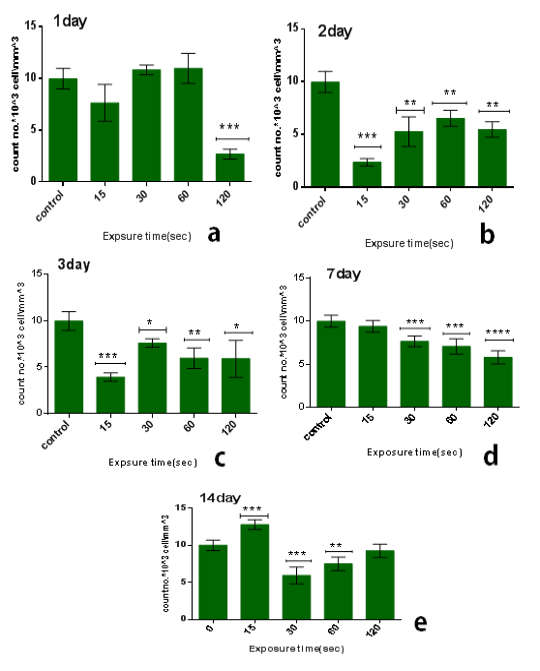
Figure 4: Effect of multi dose of CAP on the count of leukocytes against with time measured after exposure (1,2,3,7,14) day ,with stabilized dose of plasma (A): 15sec, (B): 30sec, (C) : 60sec, (D): 120sec. Data are expressed as means+SD,*p<0.05 ,**p<0 .01, ***p<0 .001,****p<0 .0001,*****p<0 .00001, (*) compared with control group.
Discussion
CAP is an important tool for medical application, especially in the treatment of cancer such as breast cancer (Yan et al., 2017), melanoma skin cancer cells (Kroesen et al., 2009). Medically, any drug or radiation to treat any disease should be studied for its effect on the body’s organs and functions. The blood is one of the most important tissues of the living body, which can be affected by cold atmospheric plasma, Therefore, we studied the effect of CAP on the number of white blood cells (in vivo) and plasma are able to penetrate 4mm of skin scaffold after 1200 seconds of exposure (Abdullah et al., 2017). So it can penetrate the body fluids up to the blood, which is transmitted to all members of the body and thus could affect the bone marrow, which is the main laboratory of blood components,and thus could affect the number od leukocytes.
To explain all the above results, the following scenario can be proposed: CAP has a direct apoptotic inflence on peripheral leukocytes in the first day or few days after exposure and this is to somehow expected from results literatures that documented apoptosis of different cell lines (Siu et al., 2015). Although the normal cells are less affected than malignant cells. At this stage, this probably indicates that CAP effect is transmitted from the skin surface through interstitial fluids to serum of blood an eventually induces its effect on leukocytes.
With blood circulating through the body of animal, reactive species created by CAP do reach bone marrow and stimulate it to produce leukocytes. This effect takes place when using single dose regimen 2 days after exposure and looks obvious after 14days and leukocyte count becomes even higher than that of control group.
In multidose regimen, nearly similar picture can be seen but bone marrow stimulation is more effective with lowest doses particularly 15sec dose at which the leukocyte count jumps far more that of control group whereas it is after 14 days with single dose regimen.
As anormal physiological regulatory mechanism, certain hormones are usually produced in serum when there is reduction in blood cells counts as a feedback mechanism stimulatingbbone marrow to produce and compensate cells loss, However, there is no evidence in this work that can exclude this physiological process so that further investigations in the future should be conducted to explore this point.
.
Conclusion
It can be concluded that CAP has some immune modulation effect which may be promising in cancer therapy including leukemia’s. Further studies are suggested to get more deep information and some room has to be given to adverse or side effects of exposure to CAP.
Acknowledgements
The authors would like to acknowledge the staff of the College of Science for Women-Department of Physics for their cooperation.
Conflict of interest
Authors declare no conflict of interests.
AUTHORS CONTRIBUTION
Ansam Q. Gadhban conducted the research, while all the authors participated in writing and proof reading of the manuscript.
References





