Advances in Animal and Veterinary Sciences
Research Article
The Nephropreventive and Antioxidant Effects of Navel Orange Peel Hydroethanolic Extract, Naringin and Naringenin in N-Acetyl-P-aminophenol-administered Wistar Rats
Osama M. Ahmed1,*, Hanaa I. Fahim1, Heba Y. Ahmed2, Basant Mahmoud3, Saad Ali Saad Aljohani4, Walaa H. Abdelazeem1
1Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef 62514, Egypt; 2Rodents Division, Department of Harmful Animals, Plant Protection Research Institute, Agriculture Research Center, Egypt; 3College of Medicine, Al-Rayyan Colleges, Al Madinah Al Munawarah, 41411, Saudi Arabia; 4Biochemistry Division, Chemistry Department, Faculty of Science, Beni-Suef University, Beni-Suef 62514, Egypt.
Abstract | The purpose of present study was to assess the nephropreventive and antioxidant effects of navel orange peel hydroethanolic extract, naringin and naringenin in N-acetyl-p-aminophenol (APAP)-administered male Wistar rats. APAP was administered at dose 0.5 g/kg/b.w. every other day by oral gavage for 4 weeks to male Wistar rats. APAP-administered rats were treated with navel orange peel hydroethanolic extract (50 mg/kg b.w.), naringin (20 mg/kg b.w.) and naringenin (20 mg/kg b.w.) every other day by oral gavage during the same period of APAP administration. The treatment of APAP-administered rats with navel orange peel extract, naringin and naringenin significantly improved the kidney function manifested by a significant decrease in the elevated serum urea, uric acid and creatinine levels. In association, the deteriorated kidney histological changes represented by congestion, hypertrophied glomerulus, vacuolization of the endothelial cells lining the glomerular tuft and interstitial nephritis in APAP-administered rats were remarkably amended. The elevated kidney lipid peroxidation and the lowered glutathione content as well as the suppressed antioxidant enzymes activities were significantly ameliorated. In conclusion, the navel orange peel hydroethanolic extract, naringin and naringenin have improvement effects on kidney function and structural integrity which may be mediated, at least in part, via enhancement of the antioxidant defense system. .
Keywords | N-acetyl-p-aminophenol, Kidney injury, Navel orange peel, Naringin, Naringenin, Oxidative stress.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 22, 2018; Accepted | August 19, 2018; Published | November 23, 2018
*Correspondence | Osama M Ahmed, Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef 62514, Egypt; Email: osamamoha@yahoo.com
Citation | Ahmed OM, Fahim HI, Ahmed HY, Mahmoud B, Aljohani SAS, Abdelazeem WH (2019). The nephropreventive and antioxidant effects of navel orange peel hydroethanolic extract, naringin and naringenin in n-acetyl-p-aminophenol-administered wistar rats. Adv. Anim. Vet. Sci. 7(2): 96-105.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.2.96.105
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
N-acetyl-p-aminophenol (APAP), acetaminophen, is one of the widely used and most popular drugs for the therapy of pain and fever (Madinah et al., 2015). Although APAP is considered as a safe drug, its overdose could result in severe liver and kidney injuries, and even death (Kandemir et al., 2017; Bektur et al., 2016). APAP is especially dangerous on the liver when it is taken over long periods (Sarumathy, 2011). Although kidney injury is less common than liver injury in APAP overdose, acute renal failure and tubular damage can occur even in the absence of liver toxicity (Madinah et al., 2015). The liver, and to a lesser extent the kidney and intestine, are the major organs implicated in the metabolism of APAP (Bessems and Vermeulen, 2001). In its biotransformation, APAP is deactivated in the liver by drug-metabolizing enzymes such as uridine diphosphate (UDP) glucuronosyltransferases (UGTs) and sulfotransferases (SULTs) to cause glucuronylation and sulfation of metabolites which are readily excreted in urine without any signs of toxicity at therapeutic doses (Mazaleuskaya et al., 2015; Kandemir et al., 2017). At high doses, a significant portion of APAP is metabolized by the cytochrome P450 system leading to the production of reactive toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI) which is detoxified by interaction with sulfhydryl groups by the reduced glutathione (GSH) (El-Shaibany et al., 2016; Abdel-Zaher et al., 2008). The NAPQI, that is not detoxified, begins lipid peroxidation and ultimately induces kidney injury (El-Shaibany et al., 2016). Therefore, the APAP toxicity is determined by the amount of the produced NAPQI and the inadequate GSH for APAP detoxification (Naguib et al., 2014). The elevated serum urea and creatinine levels are indicators of acute tubular necrosis induced by APAP (Cobden et al., 1982; Blazka et al., 1996). It was reported that free radicals produced by exposure to drug toxicity and oxidative damage in an organism plays an important role in APAP-induced hepatorenal injuries (Das et al., 2010).
Natural compounds having antioxidant activity could be used as alternative treatments of APAP toxicity (Canayakin et al., 2016). Flavonoids are naturally occurring substances that have different therapeutic applications and pharmacological action (Hamid et al., 2012). Some flavonoids due to their phenolic structures have antioxidant properties and inhibit free radical production (Topal et al., 2016). Citrus fruit extracts possess high quantity of flavonoids and exhibit potent free radical scavenging efficacy (Alam et al., 2016). Naringin and naringenin are citrus flavonoids that are strong scavengers of free radicals and prevent lipid peroxidation (Cavia-Saiz et al., 2010; Adil et al., 2016). In this regard, Dahal and Mulukuri (2015) stated that flavonoids prevent renal oxidative stress via increasing the rate of GSH by induction of its synthesis or by a scavenger effect of free radicals and reactive oxygen species (ROS).
In conductance with the previous literature, the present study aimed to evaluate the comparable nephropreventive and antioxidant effects of navel orange peel hydroethanolic extract, naringin and naringenin in APAP-supplemented male Wistar rats.
MATERIALS AND METHODS
Experimental Animals
Adult male Wistar rats weighing 130-150 g (10-12 weeks) were used in the present investigation. The animals obtained from the animal house in the National Research Center, Cairo, Egypt, were housed in good aerated cages in Animal House of Zoology Department, Faculty of Science, Beni-Suef University, Egypt at 12-hours daily light-dark cycles and temperature between 20-25°C. Animals were supplemented daily standard pelleted diet and were given water ad libitum. The animals were kept for 2 weeks under observation before the onset of the experiment to exclude any intercurrent infection. All animal methodologies followed the guidelines and were in accordance with the recommendations of the Experimental Animals Ethics Committee of Faculty of Science, Beni-Suef University, Egypt (The ethical approval number is BSU/FS/2014/4). All efforts were done to minimize the suffering, distress and discomfort of the animals.
Chemicals
APAP, naringin and naringenin were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used in this investigation were of analytical grade.
Extract Preparation
Navel orange (mutant of Citrus sinensis) fruits were obtained from local markets in Beni-Suef Governorate, Egypt. They were authenticated by Dr. Walaa A. Hasan, Lecturer of Plant Taxonomy, Department of Botany, Faculty of Science, Beni-Suef University, Egypt. Navel orange fruits were washed several times with fresh water to ensure removal of any contamination. Then, they were peeled and the peels were air dried in shade area for 20 days. The dried peels were coarsely powdered with an electrical grinder and the powder was soaked in 70% aqueous ethanol for 72 hours at room temperature. To fully mix the powder with the extraction solvent, the suspensions were allowed to be stirred frequently. The hydroethanolic extract was then filtered through Whatman filter paper and was evaporated under reduced pressure using Rotavapor to yield crude extract of navel orange peel (Mostafa et al., 2016; Ahmed et al., 2017).
Experimental Design
The animals used in this study were allocated into five groups (six rats for each) and were designed as follow:
Group 1 (Normal control group): Rats of this group were regarded as normal control group and were orally administered the equivalent volume of 1% carboxymethylcellulose (CMC), every other day for 4 weeks by oral gavage.
Group 2 (APAP group): Animals of this group were regarded as APAP control group and were orally administered APAP (dissolved in distilled water) at dose level of 0.5 g/kg b.w. every other day for 4 weeks by oral gavage (Tabassum and Agrawal, 2004).
Group 3 (APAP + navel orange peel hydroethanolic extract group): Rats of this group were administered APAP as group 2 and were treated with navel orange peel hydroethanolic extract (dissolved in 1% CMC) at dose 50 mg/kg b.w. (Mostafa et al., 2016) by oral gavage every other day for 4 weeks.
Group 4 (APAP + naringin group): Rats of this group were administered APAP as group 2 and were treated with naringin (dissolved in 1% CMC) at dose level of 20 mg/kg b.w. (Adil et al., 2016) by oral gavage every other day for 4 weeks.
Group 5 (APAP + naringenin group): Rats of this group were administered APAP as group 2 and were treated with
naringenin (dissolved in 1% CMC) at dose level of 20 mg/kg b.w. (Mershiba et al., 2013) by oral gavage every other day for 4 weeks.
Blood And Kidney Sampling
By the end of the experiment, blood was collected from jugular veins, left to coagulate at room temperature and then centrifuged at 3000 round per minute (rpm) at room temperature for 15 min. The sera were quickly separated and kept in deep freezer at -30oC pending detection of various biochemical investigations.
After blood sampling, animals were decapitated by cervical dislocation and dissected. Pieces of kidney (5 mm3) of each animal were fixed in 10% neutral buffer formalin for histopathological studies. Another 0.5 g of the kidney of each rat was homogenized in 5 ml 0.9% NaCl. The homogenate was centrifuged at 3000 rpm for 15 minutes and separated supernatants were kept in deep freezer at -30 oC pending detection of oxidative stress and antioxidant defense system markers.
Biochemical Analysis
Serum creatinine and urea levels were determined according to the methods of Fabiny and Ertingshausen (1971) and Tabacco et al. (1979) respectively using reagent kits obtained from Biosystem S.A. (Spain). Uric acid was assayed by enzymatic colorimetric method using kits obtained from Spinreact according to the method of Fossati et al. (1980).
Detection of Oxdative Stress and Antioxidant Defense System Parameters
Kidney lipid peroxidation (LPO) was detected by estimation of the formed manlondialdehyde (MDA) level according to the method of Beutler et al. (1963). Kidney glutathione (GSH) content was determined according to the method of Preuss et al. (1989). Kidney glutathione peroxidase (GPx), glutathione-S-transferase (GST) and superoxide dismutase (SOD) activities were assayed according to the methods of Matkovics et al. (1998), Mannervikand Guthenberg (1981) and Marklund, and Marklund (1974) respectively. All reagents for detection of oxidative stress and antioxidant parameters were prepared in our laboratory.
Histopathological Investigation
After dissection, kidneys from each rat were rapidly excised and then washed in saline solution by perfusion. Pieces from the kidney (5 mm3) of each rat were rapidly taken and fixed in 10% neutral buffered formalin for 24 hours. Then, they were rinsed in running tap water and serial dilutions of ethanol were used for dehydration process, cleared in xylene and embedded in paraffin at 56°C in hot air oven for 24 hours. The paraffin wax tissue blocks were prepared for sectioning by microtome at 4 µm thickness. Freshly prepared sections, floating on a 40°C water bath containing distilled water, were collected on glass slides, deparaffinized, stained with hematoxylin and eosin (H&E) stains according to the method of Banchroft et al. (1996).
Statistical Analysis
All data were expressed as means ± standard error (SE). Statistical analysis was done using Statistical Package for Social Sciences (SPSS) computer software (version 22), IBM software, USA. One-way analysis of variance (ANOVA) test was used to elucidate significance among group means, followed by Tukey’s post-hoc test and least significance difference (LSD) to compare mean values pair-wise. Differences were considered significant at p<0.05.
RESULTS
The serum creatinine, urea and uric acid levels were significantly elevated (P>0.05) in APAP-administered rats recording percent changes of 83.01, 168.23 and 92.59 % respectively as compared with normal control rats. The treatment of APAP-administered rats with navel orange peel extract produced a significant (P>0.05) amelioration of the elevated creatinine, urea and uric acid levels recording percent changes of -45.36 -58.84 and -53.52 % respectively as compared with APAP-administered rats. Similarly, the treatment with naringin and naringenin significantly (P>0.05) decreased the elevated serum creatinine, urea and uric acid levels. The peel extract was the most potent in improving the elevated creatinine level (Table 1).
Kidney LPO of APAP-administered rats was significantly increased (P>0.05) recording percentage change of 53.19% as compared with normal control. Conversely, GSH level was significantly declined (P>0.05) recording -34.85% as compared with normal control. The treatment of APAP-administered rats with peel extract produced a significant decrease in kidney LPO (-22.16%) and a significant increase in kidney GSH content (16.46%) as compared with APAP–administered control rats. Similarly, the naringin and naringenin treatment significantly ameliorated the elevated LPO and the lowered GSH content as compared with APAP-administered control. The peel extract was most potent in improving the elevated LPO (-22.16%) while naringenin was the most effective in alleviating the lowered kidney GSH content (+22.10%) (Table 2).
APAP administration results in a marked impairment in kidney antioxidant enzyme activities as demonstrated by the significant decline (P>0.05) in GST, GPx and SOD activities. As a result of treatment of APAP-administered
Table 1: Effects of navel orange peel ethanolic extract, naringin and naringenin on serum urea, uric acid and creatinine levels of acetaminophen-administered albino rats.
| Parameter Groups | Creatinine (mg/dl) | % Change |
Urea (mg/dl) | % Change |
Uric acid (mg/dl) | % Change |
| Normal | 0.53 ± 0.042 |
- | 25.66 ± 1.49 |
- | 1.62 ± 0.143 |
- |
| APAP | 0.97 ± 0.031a |
83.01 | 68.83 ± 1.01a |
168.23 | 3.12 ± 0.104a |
92.59 |
| APAP + navel orange peel extract | 0.53 ± 0.042b |
-45.36 | 28.33 ± 2.10b |
-58.84 | 1.45 ± 0.088b |
-53.52 |
| APAP + naringin | 0.56 ± 0.036b |
-42.26 | 29.16 ± 2.31b |
-57.63 | 1.36 ± 0.114b |
-56.41 |
| APAP + naringenin | 0.58 ± 0.038b |
-40.20 | 28.83 ± 2.52b |
-58.11 | 1.21 ± 0.147b |
-61.22 |
Data were expressed as mean ± SE. - Number of animals in each group was 6.
a Significantly different from normal value at p < 0.05, b Significantly different from APAP value at p < 0.05.
Percentage (%) changes were calculated by comparing APAP-administered rats with normal control and APAP-administered treated rats with APAP-administered rats.
Table 2: Effects of navel orange peel extract, naringin and naringenin on LPO and GSH level in kidney of acetaminophen-administered albino rats.
| Parameter Groups | LPO (nmol /100mg tissue) | %Change | GSH (nmol/100mg tissue) | %Change |
| Normal | 69.65± 1.73 |
- | 26.11 ± 0.50 |
- |
| APAP | 106.70 ± 1.25a |
53.19 | 17.01 ± 0.45a |
-34.85 |
| APAP + navel orange peel extract | 83.05 ± 4.64b |
-22.16 | 19.81 ± 0.60ab |
16.46 |
| APAP + naringin | 85.98 ± 4.07ab |
-19.42 | 19.25 ± 0.64ab |
13.16 |
| APAP + naringenin | 87.87 ± 4.23ab |
-17.65 | 20.77 ± 0.90ab |
22.10 |
Data were expressed as mean ± SE. - Number of animals in each group was 6.
a Significantly different from normal value at p < 0.05, b Significantly different from APAP value at p < 0.05.
Percentage (%) changes were calculated by comparing APAP-administered rats with normal control and APAP-administered treated rats with APAP-administered rats.
Table 3: Effects of navel orange peel extract, naringin and naringenin on GST, GPx and SOD activities in kidney of acetaminophen-administered albino rats.
| Parameter Groups | GST (U/100mg tissue) |
% Change | GPx (mU/100 mg tissue) | %Change | SOD (mU/100mg tissue) | %Change |
| Normal | 109.50 ± 2.32 |
- | 154.78 ± 1.27 |
- | 98.57± 0.97 |
- |
| APAP | 90.79 ± 2.34a |
-17.08 | 144.55 ± 1.10a |
-6.61 | 79.27 ± 1.22a |
-19.57 |
| APAP + navel orange peel extract | 101.24 ± 3.41 |
11.51 | 149.80 ± 0.50ab |
3.63 | 73.29 ± 1.46a |
-7.54 |
| APAP + naringin | 101.94 ± 3.25 |
12.28 | 151.43 ± 0.87b |
4.75 | 83.28 ± 2.36a |
5.05 |
| APAP + naringenin | 104.28 ± 2.69b |
14.85 | 149.65 ± 1.38ab |
|
90.77 ± 2.33ab |
14.50 |
Data were expressed as mean ± SE. - Number of animals in each group was 6.
a Significantly different from normal value at p < 0.05, b Significantly different from APAP value at p < 0.05.
Percentage (%) changes were calculated by comparing APAP-administered rats with normal control and APAP-administered treated rats with APAP-administered rats
rats with the peel extract, GST and GPx activities were remarkably increased; the recorded percentage changes were 11.51 and 3.63% respectively as compared with APAP-administered rats. Furthermore, the treatment of APAP-administered rats with naringin and naringenin markedly increased the GST, GPx and SOD activities. However, while the effect of navel orange peel and naringin on kidney GPx activity was significant (P>0.05), the effect of naringenin was significant on GST, GPx and SOD activities. The naringenin was the most effective in increasing the declined activities of GST and SOD while naringin was the most potent treatment in improving GPx activity (Table 3).
Histological Changes
Kidney sections of normal rats demonstrated the normal histoarchitecture of the glomerulus and surrounding tubules, proximal and distal tubules (Figure 1; Photomicrograph 1a). Oral administration of APAP caused several lesions in kidney tissue including congestion of renal blood vessel and congestion of glomerulus (Figure 1; Photomicrograph 1b). It also produced hypertrophy of the glomerulus and vacuolization of the endothelial cells lining the glomerular tuft (Figure 1; Photomicrograph 1c) as well as interstitial nephritis (Figure 1; Photomicrograph 1d). On the other hand, the treatment of the APAP-administered rats with navel orange peel hydroethanolic extract improved APAP-induced kidney histological lesions and the kidney attained its normal histological structure with normal glomerulus and tubules (Figure 2; Photomicrographs 2a & b). The same ameliorative effects appeared after treatments with naringin and naringenin (Figures 3 and 4).
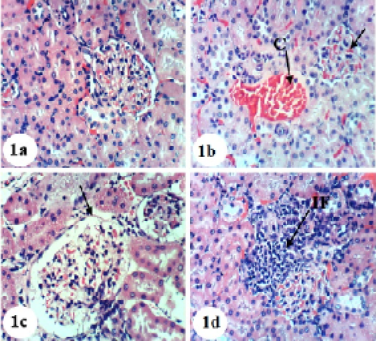
Figure 1: Photomicrographs of H&E stained kidney sections of normal and APAP-administered rats. 1a: Photomicrograph of kidney section of normal rats showing normal histologic structure of kidney; normal glomerulus (G), normal proximal tubules (Pt) and distal tubules (Dt) 1b-d: Photomicrograph of kidney section of APAP-administered rats showing congestion of renal blood vessel (C), congestion of glomerulus (CG) in b, hypertrophied glomerulus (HG), vacuolization of the endothelial cells lining the glomerular tuft (V) in c and interstitial nephritis (IF) in d. ×400
In spite of these alleviative effects of navel orange peel hydroethanolic extract, naringin and naringenin, there are still slight congestion in the glomeruli and intertubular blood vessels.
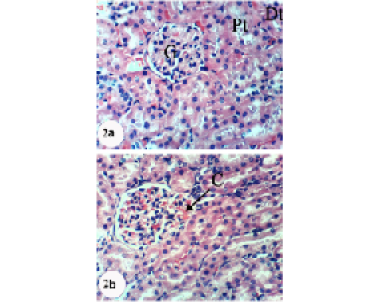
Figure 2: Photomicrographs of H&E stained kidney sections of APAP-administered rats treated with navel orange peel hydroethanolic extract. 2a: Photomicrograph of kidney section showing normal structure of kidney; normal glomerulus (G), proximal (Pt) and distal tubules (Dt). 2b: Photomicrograph of kidney section showing slight congestion of glomerulus (CG). X400
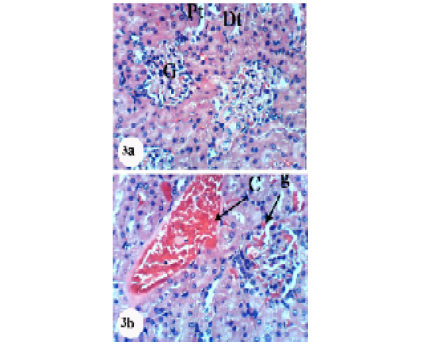
Figure 3: Photomicrographs of H&E stained kidney sections of APAP-administered rats treated with naringin. 3a: Photomicrograph of kidney section showing normal structure of kidney; normal glomerulus (G), proximal (Pt) and distal tubules (Dt). 3b: Photomicrograph of kidney section showing congestion of renal blood vessel (C) and congestion of glomerulus (CG). ×400
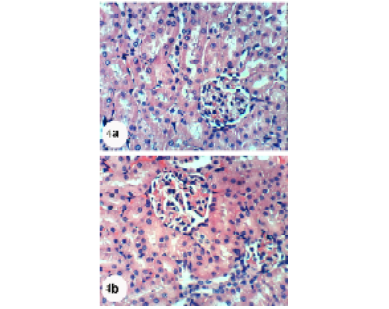
Figure 4: Photomicrographs of H&E stained kidney sections of APAP-administered rats treated with naringenin showing normal glomerulus (G), proximal tubule (Pt) and distal tubule (Dt). Slight glomerular congestion (GC)and intertubular congestion (C) were observed in 4b. X400
DISCUSSION
Kidneys play an essential role in animals and humans (Kandemir et al., 2017). They remove substances from the body including various toxins, metabolic products and other foreign substances such as food additives, drugs and pesticides (Wudil and Sarki, 2015). APAP overdose remains to be the most important cause of toxicity in many parts of the world among all drug toxicities (Karakus et al. 2013; Yayla et al., 2014; Canayakin et al., 2016). Hepatotoxic and nephrotoxic effects of APAP overdose occur by a complex sequence of events (Hinson et al. 2010). APAP-induced nephrotoxicity becomes evident after hepatotoxicity in most cases but the occurrence of renal tubular damage and acute renal failure, even in the absence of liver injury, should not be ignored (Eguia & Materson, 1997).
Oral administration of APAP in the present study, at dose of 0.5 g/kg/b.w. every other day for 4 weeks, showed significant increase in serum levels of creatinine, urea and uric acid. These results are in parallel with those of various past publications (Brune et al., 2015; Adil et al., 2016 and Oseni et al., 2017).
Increase in serum urea level is an important marker of renal toxicity (Kandemir et al., 2017). Kidney disease and function disorders may be manifested by serum urea accumulation exceeding its clearance rate (Oseni et al., 2017). Similarly, increase in plasma creatinine level is considered as kidney dysfunction biomarker (Yousef et al., 2010). Overdoses of APAP cause many metabolic perturbances including an increase in serum urea and creatinine (Srinivasan et al., 2014; Ghosh et al., 2010). These changes occurred as a result of the inactivation of the mitochondrial pathway during APAP-induced cell death (Ijaz et al., 2016). In addition, Ajami et al. (2010) explained the increase in the urea and creatinine levels by the presence of strong correlation between kidney injury and oxidative stress. They also stated that the elevated ROS production may alter the filtration surface area and modify the filtration coefficient; this could decrease the glomerular filtration leading to accumulation of creatinine and urea in the blood.
The increase in serum creatinine, urea and uric acid levels due to APAP ingestion in the current study were correlated closely with the histopathological changes in the kidney. These histopathological alterations include congestion of glomerular tuft and renal blood vessel, glomerular hypertrophy, vacuolization of glomerular tuft cells and interstitial nephritis.
The treatment of rats with navel orange peel hydroethanolic extract for 4 weeks, in this study, significantly diminished the elevated serum levels of kidney function parameters including creatinine, urea and uric acid and ameliorated kidney histological architecture and integrity. In accordance with present study, Ahmad et al. (2012) reported that APAP induced elevation in serum creatinine and urea levels, but co-treatment with extract of orange peel substantially decreased the serum creatinine and urea levels.
In the current study, the levels of creatinine, urea and uric acid were significantly decreased by naringin treatment as compared to APAP control rats in association with reduced histological deteriorations in the kidney. These results are in line with those of Adil et al. (2016). Naringenin administration also decreased the elevated levels of kidney function parameters. In parallel with this finding, Hermenean et al. (2013) indicated the ability of naringenin to protect the kidney against CCl4-induced renal toxicity in male Swiss mice.
The nephroprotective effect of citrus by-products extracts may be due to presence of phyto-constituents like polyphenolic compounds, especially the characteristic flavanone glycosides which mainly include hesperidin, neohesperidin, naringin, rutin and narirutin (Alam et al., 2014). This attribution may explain the most potent effects of navel orange peel hydroethanolic extract on the elevated serum creatinine level and kidney histological structure and integrity.
Ingestion of APAP to rats induced a disturbance in the oxidant/antioxidant status characterized by a significant increase in kidney LPO and decrease in GSH content as well as GPx, GST and SOD activities. These findings are in accordance with those of many published works (Canayakin et al., 2016; Ijaz et al., 2016; Kandemir et al., 2017). The stimulated oxidative stress may be mediated, at least in part, through accumulation of the toxic metabolite NAPQI, a metabolite of APAP that has high affinity for GSH leading to its depletion (Amin et al., 2017).
Kidney LPO was manifested by detection of MDA production. MDA is known as a secondary product of LPO and is used as a marker of oxidative tissue damage resulting from many chain reactions (Gülçin and Beydemir, 2013). Some previous studies have stated that renal oxidative stress is induced by increases in LPO products (e.g., MDA) and suppression in antioxidant defensive system (Gebaly et al., 2012). Elevated MDA levels in kidney and other tissues have long been known to cause functional degradation; thereby, the degradation of vital tissue leading to complications may be indirectly due to increased oxidative stress and production of ROS (Pushpavalli et al., 2010).
GSH is a non-enzymatic antioxidant that protects the tissues and organs against the adverse effects of ROS. It plays a crucial role in eliminating free radical species such as H2O2, superoxide radicals and membrane protein thiols (Bursal et al., 2013). It is also known as a substrate of the GPx enzyme (Basu et al., 2012). In therapeutic doses, APAP directly establishes a conjugated bond with glucuronic acid and sulphate, forming non-toxic conjugated metabolites that are eliminated by the kidneys (Gamal El-Din et al., 2003). On the other hand, high doses of APAP lead to tissue damage and stimulate programmed necrosis or apoptosis, as well as deteriorating homeostasis, and ultimately induce mitochondrial function disorders (Wudil and Sarki, 2015). Antioxidant such as SOD and GSH are the first line defense system that confines the toxicity allied with the free radicals (Saraswathi et al., 2014).
Enzymatic and non-enzymatic antioxidants play an important role by protecting the cells exposed to oxidative damage (Canayakin et al., 2016). The increase in LPO was accompanied by a significant reduction in the level of GSH which is an important free radical and NAPQI scavenger (Yayla et al., 2014) and plays a central role in the antioxidant defense system (Bursal et al., 2013).
The decreased SOD and GSH levels and the increased LPO in the APAP-administered rats, in present study, were in accordance with previous research studying APAP-induced nephrotoxicity (Ghosh and Sil, 2007; Yousef et al., 2010; Abdul Hamid et al., 2012; Naguib et al., 2014; Hommore et al., 2015).
In the current study, the treatment of APAP-administered rats with navel orange peel hydroethanolic extract for 4 weeks significantly decreased the renal LPO and remarkably increased the renal GSH content and GST and GPx activities towards the normal control values. These effects might be due to the antioxidant potential of citrus peel hydroethanolic extract that contains many antioxidant phytochemicals like naringin, hesperidin, neohesperidin and narirutin (Anagnostopoulou et al., 2006). These results were confirmed with the observation of Mostafa et al. (2016).
Similarly, the treatment with naringin and naringenin, in the current study, significantly improved the APAP-induced elevation of kidney LPO and APAP-induced deteriorated effects on GSH content and GST, GPx and SOD activities. While navel orange peel extract was the most effective in suppressing kidney LPO, naringenin was the most potent in ameliorating kidney GSH content as well as GST and SOD activities. Thus, these results suggest that navel orange peel extract, naringin and naringenin have potential to suppress the oxidative stress and enhance the antioxidant defense system in kidneys. These results are in accordance with many previous publications. Guimarães et al. (2010) stated that citrus fruit extracts possess large amounts of flavonoids and show potent free radical scavenging activity. Cavia-Saiz et al. (2010) reported that both naringin and naringenin are strong scavengers of free radicals and prevent LPO. Anandhi et al. (2013) revealed that naringin possesses potent antioxidant, anti-free radical scavenging and metal chelating properties. Mostafa et al. (2016) found that the activities of antioxidant enzymes glutathione reductase (GR), GST, SOD and catalase (CAT) were markedly restored by pretreatment of rats with sweet orange and mandarin peel extracts, as compared to the group administered APAP alone. Hermenean et al. (2013) demonstrated that the pre-treatment with naringenin resulted in the return of antioxidant and renal protective effects against injuries induced by CCl4
The improvement in the antioxidant defense system as a result of treatments of APAP-administered rats with peel hydroethanolic extract, naringin and naringenin, in the present study, was associated with amendment of the kidney histological integrity and architecture; this reflects the role of antioxidant defense system in mediating the ameliorative effects of these treatments on the kidney. Naringin as well as navel orange peel extract and naringenin could maintain the potency of the antioxidant defense system, which in turn modulate the membrane integrity against APAP-induced cellular injury (Adil et al., 2016).
CONCLUSION
Navel orange peel hydroethanolic extract, naringin and naringenin induced potent nephropreventive effects which were evidenced by amelioration of the kidney function parameters and histological integrity. These nephropreventive effects may be mediated, at least in part, via suppression of oxidative stress and enhancement of antioxidant defense system.
ACKNOWLEDGEMENTS
The authors sincerely acknowledged Dr. Walaa A. Hasan, Assistant professor of plant Taxonomy, Botany Department, Faculty of Science, Beni-Suef University, Egypt for her assistance in recognition and classification of the plant. The authors also would like to express their sincere appreciation to Prof. Dr. Kawkab Abd El Aziz Ahmed, Professor of Pathology, Pathology Department, Faculty of Veterinary Medicine, Cairo University and Dr. Rasha R. Ahmed, Professor of Molecular Cell Biology, Zoology Department, Faculty of Science, Beni-Suef University, Egypt for their great help in the examination of liver sections and description of histopathological changes.
COnflict Of INTERESTS
The authors declare that they have no competing interests.
authors contribution
Osama M. Ahmed proposed the research plan, guided the experimental work and shared in writing and revising the manuscript. Hanaa I. Fahim revised the manuscript and also supervised the experimental work. Heba Y. Ahmed and Basant Mahmoud participated in following up the experimental work and shared in writing and revising the manuscript. Saad Ali Saad Aljohani participated in writing and revising the manuscript. Walaa H. Abdelazeem performed the experimental work and participated in writing the manuscript.
REFERENCES





