Advances in Animal and Veterinary Sciences
Research Article
Citrus limon and Paradisi Fruit Peel Hydroethanolic Extracts Prevent the Progress of Complete Freund’s Adjuvant-Induced Arthritis in Male Wistar Rats
Osama Mohamed Ahmed, Mohamed Badr Ashour, Hanaa Ibrahim Fahim, Noha Abdelazeem Ahmed*
Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt.
Abstract | This study was designed to assess the preventive effect of Citrus limon (C. lemon) and Citrus paradisi (C. paradisi) fruit peel hydroethanolic extracts on the progress of complete Freund’s Adjuvant (CFA)-induced arthritis in male Wistar rats. Rheumatoid arthritis rat model was established by subcutaneous injection of 0.2 ml CFA into the right hind paw of each rat at two successive days. Arthritic rats were administered C.limon and C. paradisi fruit peel hydroethanolic extracts by oral gavage of 100 mg/kg body weight /day for 9 and 18 days. Administration of C. limon and C. paradisi fruit peel hydroethanolic extracts for 9 and 18 days, significantly reduced the CFA-induced paw swelling and edema in arthritic rats as manifested by a significant decrease in right hind paw circumference, volume and thickness. The increased mRNA expression of right ankle joint pro-inflammatory and inflammatory mediators including cyclooxygenase-1 (COX-1), interleukin-1β (IL-1β) and interleukin-6(IL-6) and the decreased mRNA expression of anti-inflammatory cytokine(IL-4) were significantly improved in arthritic rats by oral administration of C. limon and C. paradisi fruit peel hydroethanolic extracts. In association, the deleterious histological alterations represented by articular joint inflammatory cells infiltration, fibroblasts proliferation, edema, synovial hyperplasia, pannus formation and cartilage necrosis as well as spleen haemorrhage and lymphocytic necrosis and thymus lymphoblasts proliferation were noticeably alleviated by oral supplementation of arthritic rats with C. limon and C. paradisi fruit peel hydroethanolic extracts for 9 and 18 days. The study suggested that C. limon and C. paradisi fruit peel hydroethanolic extracts have anti-arthritic potentials which may be mediated via their anti-inflammatory activities.
Keywords | Rheumatoid arthritis, Citruslimon, Citrus paradisi, Fruit peel, Hydroethanolic extracts.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 22, 2018; Accepted | August 31, 2018; Published | September 21, 2018
*Correspondence | Noha Abdelazeem Ahmed, Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt; Email: drnohascience@science.bsu.edu.eg
Citation | Ahmed OM, Ashour MB, Fahim HI, Ahmed NA (2018). Citrus limon and paradisi fruit peel hydroethanolic extracts prevent the progress of complete freund’s adjuvant-induced arthritis in male wistar rats. Adv. Anim. Vet. Sci. 6(10): 443-455.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.10.443.455
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disorder that is characterized by inflammation of the synovial joints (Majeed and Borole, 2015; Ahmed et al., 2017) and concomitant degeneration of articular cartilage and bone (Rathore et al., 2015; Ahmed et al., 2017). The worldwide prevalence of RA is 0.5–1% of the population (Mohamed et al., 2014; Ahmed et al., 2017). RA affects female more than male at a ratio of 3:1 and it increases with the age (Scott and Wolfe, 2010). The cause of RA is multifactorial and it is thought that genetic, immunological and environmental factors all of which contribute to the disease (Shaw et al., 2014). Thus, the disease develops as a result of the effect of instigating environmental insults on a susceptible phenotype, thereby leads to immunological dysfunction characterized by activation of Th1 and Th17 and long-term inflammation (Firestein, 2003; Ahmed et al., 2017).
Animal arthritic models that reproduce the pathology of RA inhuman beings are of great interest as vehicles for the testing of potential therapeutics that may produce similar effects on human RA. These experimental models are essential to evaluate the effectiveness and safety of huge number of new potential RA therapeutic agents (Tag et al., 2014). Populations with RA-like disorder are created by complete Freund’s adjuvant (CFA) and collagen-induced arthritis (CIA) which behaves similar to human RA, creating synovitis and articular cartilage and bone erosions (Hegen et al., 2008). The similarities in joint pathology between adjuvant induced arthritis (AIA) and human RA could allow screening of new drugs for treatment of RA disease (Lorenzo et al., 2008).
Conventional medicine of RA includes treatment with steroids, non-steroidal anti-inflammatory drugs (NSAIDs), many biological agents such as interleukin-1beta (IL-1β) and tumor necrosis factor alpha (TNF-α) antagonists (Fleischmann et al., 2004), and disease-modifying anti-rheumatic drugs (DMARDs) (Choy et al., 1998). These treatment agents are associated with adverse side effects (Scheiman, 2001) and have shown only limited success in all forms of arthritis (Chandrashekara et al., 2002; Tag et al., 2014). Thus, alternative therapies of RA such as treatment with herbal products and plant extracts are receiving increasing public and research interests (Soeken et al., 2003). Fruits and vegetables have been recognized as natural sources of various bioactive constituents and drugs (Pennington and Fisher, 2010; Dembitsky et al., 2011). The main phytochemical constituents present in fruits and vegetables are flavonoids, phenolic compounds, anthocyanins, carotenoids, vitamins C and E and dietary fiber (Gonzalez-Aguilar et al., 2008).
Citrus limon (C. limon), lemon, is a rich source of bioflavonoids (mostly from pith and peel)and vitamin C and potassium (Bhavana et al., 2016). Other constituents of lemon include volatile oil (2.5% of the peel), limonene, alpha-pinene, alpha-terpinene, citral, coumarins, mucilage and pectins (Fisher and Phillips, 2006; Miyake et al., 2007). The pectin fiber as well as lemon oil have antioxidant potentials (Polunin, 1997; Tag et al., 2014).
Citrus paradisi (C. paradisi) fruit, grapefruit, are storehouse of vitamins such as thiamine, riboflavin, vitamin C, E, niacin and pantothenic acid (Jaffe, 2013). It has a high content of dietary fiber too and it is an excellent source of folic acid and minerals. Researchers have shown that grapefruit has positive effect in reducing the inflammation and pain in joints of arthritis patients mainly in osteoarthritis due to presence of its various nutritional components (Ganzera et al., 2006; Agarwal, 2013).
Therefore, this study was conducted to assess the effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on the inflammatory status as well as the histological architecture of ankle joint, spleen and thymus of CFA-induced arthritic Wistar rats.
MATERIAL AND METHODS
Experimental Animals and Housing
Adult male Wistar rats weighing 130-150 g were obtained from Helwan Station of Experimental Animals, Egyptian Organization for Biological Products and Vaccines (VACSERA), Helwan, Cairo, Egypt. They were overseen for two weeks before the initiation of the experiment as an acclimatization period and to exclude any intercurrent infection. The animals were housed in polypropylene cages with stainless-steel metal good aerated covers in the Animal House of Zoology Department, Faculty of Science, Beni-Suef University, Egypt at normal temperature (20-25°C) and normal day/night cycle (10-12h/day) and were given free access of balanced standard diet and water. All animal procedures followed the standard guidelines of care and use of animals of the Experimental Animal Ethics Committee of Faculty of Science, Beni-Suef University, Beni-Suef, Egypt (Ethical Approval number: BSU/FS/2017/20). All efforts were made to minimize the animal pain, distress and discomfort.
Induction of Arthritis
CFA, which is a suspension of heat-killed Mycobacterium tuberculosis in mineral oil (each 1 ml CFA contains 1 mg Mycobacterium tuberculosis), was obtained from Sigma Chemical Company (St. Louis, MO 63178 USA) and was used to induce RA in male Wistar rats. The arthritis was induced by a double subcutaneous injection of 0.2 ml CFA, into the footpad of the right hind leg of rats in two consecutive days (0.1 ml/day) according to the previous publication (Ahmed et al., 2017).
Extraction of Lemon and Grapefruit Peels
C.limon and C. paradisi fruits were obtained from Beni-Suef Governorate local market. They were authenticated by Dr. Walaa A. Hassan, Lecturer of Flora, and the Collections Manager of Herbarium of Botany Department, Botany Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt. The fruits were washed with tap water and peeled. The excised peels were washed using current tap water and finally with distilled water. Then, they were air-dried in shade. The dried peels were powdered with an electric grinder and extracted by cold maceration in 70% aqueous ethyl alcohol at room temperature. After filtration, the filtrates were concentrated by using rotary evaporator under a vacuum system with reduced pressure within the evaporator system. The dry filtrate obtained was stored at -20oC until used for the experimental evaluations.
Dose Preparation of Lemon and Grapefruit Peel’s Ethanolic Extract
A 100 mg of either C. limon fruit peel hydroethanolic extractor C. paradisi fruit peel hydroethanolic extract was dissolved in 5 ml of 1% carboxy methylcellulose (CMC) solution which was used as a vehicle and was orally given by oral gavage to kg body weight (b.w.) of rats daily for 18 days. The dose of C. limon peel hydroethanolic extract (100mg/kg b.w.) was chosen according to (Tag et al., 2014) while that of C. paradisi peel hydroethanolic extract (100mg/kg b.w.) was chosen according to Mossa et al. (Gupta et al., 2011; Mossa et al., 2015).
Animal Grouping
The rats used in this experiment were allocated into 4 groups, each being 12 rats. Six rats of each group were sacrificed after 9 days and the other six after 18 days. These groups are designated as follow:
Group 1 (Normal group): The rats included in this group received an equivalent volume of 1% CMC daily by oral gavage for 18 days beginning from the starting period of the experiment.
Group 2 (Arthritic control group):The rats included in this group received a double subcutaneous injection of 0.1 ml CFA/day, into a footpad of the right hind leg in two consecutive days and were administered equivalent volume of 1% CMC daily by oral gavage for 18 days beginning from the starting period of the experiment.
Group 3 (Arthritic group treated with C. limon fruit peel extract): The rats of this group received a double subcutaneous injection of 0.1 ml CFA, into a footpad of the right hind leg in two consecutive days as group 2 and were supplemented with 100 mg/kg b.w./day C. limon fruit peel hydroethanolic extract dissolved in 5ml 1% CMC by oral gavage throughout the entire experimental period (18 days).
Group 4 (Arthritic group treated with C. paradisi fruit peel extract): The rats of this group received a double subcutaneous injection of 0.1 ml CFA, into a footpad of the right hind leg in two consecutive days as group 2 and were supplemented with 100 mg/kg b.w./day C. paradisi fruit peel hydroethanolic extract dissolved in 5 ml 1% CMC by oral gavage throughout the entire experimental period (18 days).
Detection of Right Paw Circumference as Indicator of Paw Edema
After 9 and 18 days, the right hind paw circumference just above tarsal pad was measured as an indicator of swelling rate and paw edema in different groups by wrapping a piece of white cotton thread around the paw just above tarsal pad, and measuring the circumference on a meter ruler (Olajide et al., 2009; Ahmed et al., 2015; Ahmed et al., 2017).
Detection of Right Hind Paw Volume
After 9 and 18 days, the volume of paw of right hind leg was measured by putting right hind paw in a graduated falcon tube filled with saline (0.9% NaCl) at known volume and the overflow of saline which express the paw swelling was measured.
Measurement of Right Hind Paw Thickness of Inflamed Articulation
After 9 and 18 days, the right hind paw thickness was regularly measured for each with a micrometer screw gauge at 9 and 18 days of CFA injection at the mid-sagittal plane (Li et al., 2010).
Detection on Messenger Rna (Mrna) Expression of Various Cytokines
Ribonucleic acid (RNA) isolation: Total RNA was extracted from articular joints by using Thermo Scientific GeneJET RNA extraction kit obtained from Thermos Fisher Scientific Inc., Rochester, New York, USA (Chomczynski and Sacchi, 1987). Ankle with articular tissues were homogenized and lysed in lysis buffer solution that contains guanidine thiocyanate, a chaotropic salt that protects RNA from endogenous RNases. The resulting lysate was then mixed with ethyl alcohol and was loaded on a purification column. The chaotropic salt and ethyl alcohol cause RNA to bind to the silica membrane as the lysate is spun through the column. Impurities were subsequently removed from the membrane by washing the column with washing buffer solution. Pure RNA was then eluted under low ionic strength conditions with nuclease-free water.
The amount of purified RNA was quantified by using UV Spectrophotometer according to the following formula: RNA μg/μl= O.D.260nm X (40μg RNA /ml) × dilution factor/1000. The purity of RNA was checked and should be ranged between 1.8 and 2.0 to ensure the high purity of the isolated RNA. Then, 0.5 μg of purified RNA was used for production of complementary deoxyribonucleic acid (cDNA).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis: cDNA was produced from the extracted RNA and was amplified by Thermo Scientific Verso 1-Step RT-PCR ReddyMix kit obtained from Thermo Scientific Inc, Rochester, New York, USA to detect the mRNA expression of cyclooxygenase-1 (COX-1), IL-1β, interleukin-6 (IL-6) and interleukin-4 (IL-4) in articular joints using specific primers (Table 1).
The final reaction volume was 50 μl. The reaction mixture consists of Verso Enzyme Mix (1 μl), 2X 1-Step PCR Reddy Mix (25 μl), RT Enhancer (2.5 μl), sense primer (10 μM) (1 μl), anti-sense primer (10 μM) (1 μl), template RNA (2 μl) and nuclease-free water (17.5 μl).
The reaction PCR tubes were placed in a double heated led thermal cycler, and the reaction series included verso inactivation at 95oC for 2 min followed by 35 cycles initial denaturation for 20 sec at 95oC, at 50-60oC for 30 sec and at 72oC for 1 min followed by 1 cycle at 72oC for 5 min. PCR products were separated on agarose gel (1.5%) by electrophoresis. cDNA bands were observed using UV transilluminator in dark chamber. Gel images were analyzed by scanning densitometry (Gel Doc. advanced ver 3.0), and the relative expressions of COX-1, IL-1β, IL-6 and IL-4 normalized to β-actin were calculated.
Table 1: The sequences of forward and reverse primers of β-actin and various cytokines
|
Gene |
Primer Sequence |
|
β-actin (housekeeping gene) |
F: 5`TCACCCTGAAGTACCCCATGGAG3` R: 5`TTGGCCTTGGGGTTCAGGGGG3` |
|
COX-1 |
F: 5`CCTTCTCCAACGTGAGCTACTA 3ʹ R: 5`TCCTTCTCTCCTGTGAACTCCT 3` |
|
IL-1β |
F: 5`GGCAGTGTCACTCATTGTGG3´ R 5`AGGTGCTTGGGTCCTCAT 3´ |
|
IL-6 |
F: 5`GCCTTCTTGGGACTGATG3´ R: 5` TGGTCTGTTGTGGGTGGT3` |
|
IL-4 |
F: 5`GGAACACCACGGAGAACG3´ R: 5`GCACGGAGGTACATCACG3´ |
Histopathological Investigations
After sacrifice and dissection, ankle joint (decalcified in 10% ethylene diamine tetra-acetic acid [EDTA] for a period of 2–3 weeks) and pieces from spleen and thymus of rats in different groups were fixed in 10% neutral buffered (pH 7) formol saline for 48 hrs. Washing was done in tap water. Then, serial dilutions of alcohol (methanol, ethanol and absolute ethanol) were used for dehydration. For wax blocking, specimens were cleared in xylene and embedded in paraffin in hot air oven for 24 hrs. Paraffin wax tissue blocks were prepared for sectioning at 4 μm thicknesses by using rotary microtome. The obtained tissue sections were mounted on glass slides, deparaffinized and stained with hematoxylin and eosin (H&E) (Banchroft et al., 1996). The stained sections were examined for detection of lesions using light microscope with the help of histopathologist and histologist.
Statistical Analysis
Statistical analysis of the data was performed using SPSS program v.20 software (SPSS Inc., Chicago, USA). Results were expressed as mean ± standard error (SE) and all statistical comparisons were made by Tukey᾽s test post hoc analysis. Values of P<0.05 was considered significant while those of P>0.05 were considered non-significant (Nie et al., 1970).
RESULTS
The changes in the right hind paw circumference, volume and thickness as markers of paw swelling and edema were respectively illustrated in figures 1, 2 and 3. The data of CFA-induced arthritic rats depicted a significant increase (p<0.05) in the right hind paw circumference, volume and thickness after 9 and 18 days of CFA injection in comparison with the normal control group (Figures 1, 2 and 3). The treatment of arthritic rats with C. limon and C. paradisi fruit peel hydroethanolic extracts produced a significant amelioration (p<0.05) of the increased right hind paw circumference and thickness after 9 and 18 days (Figures 1 and 3). While the treatments of arthritic rats with C. limon and C. paradisi fruit peel hydroethanolic extracts induced a non-significant effect (p>0.05) on the volume of right hind paw at 9th day, they produced a significant decrease (p<0.05) at the 18th day (Figure 2).
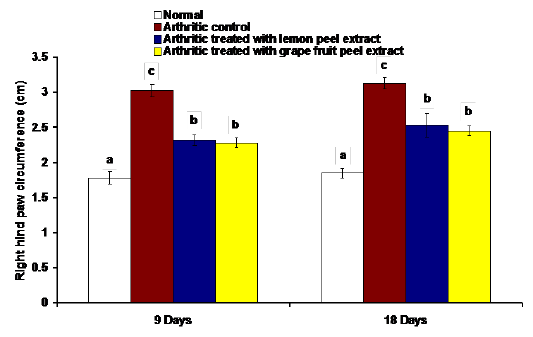
Figure 1: Effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on right hind paw circumference at tarsal pad in arthritic rats. Means, which share the same superscript symbol(s), are not significantly different.
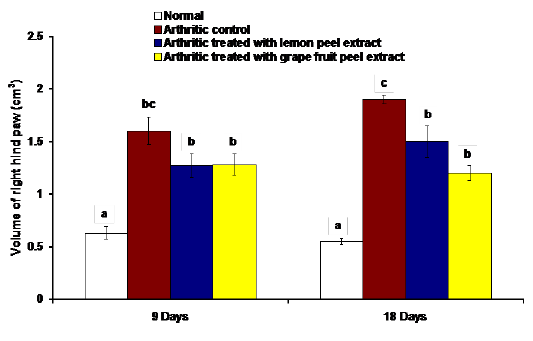
Figure 2: Effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on volume of right hind paw (cm3) in arthritic rats. Means, which share the same superscript symbol(s), are not significantly different.
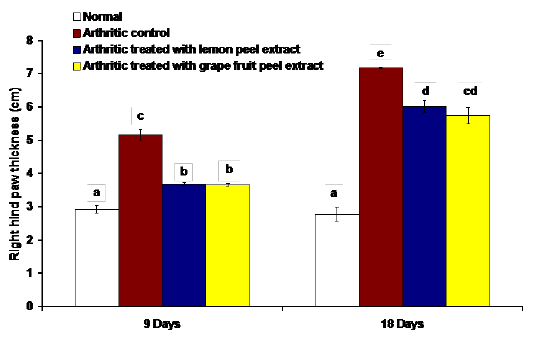
Figure 3: Effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on right hind paw thickness in arthritic rats. Means, which share the same superscript symbol(s), are not significantly different.

Figure 4: Effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on right hind leg ankle joint COX-1 expression relative to β-actin in CFA-induced arthritic rats. Means, which share the same superscript symbol(s), are not significantly different.Top: Gel photograph depicting COX-1 and β-actin PCR products Bottom: Corresponding intensity analysis of right ankle joint COX-1 PCR products from 3 different experiments, represented as the COX-1 mRNA percentage of β-actin mRNA.
The effects on the mRNA expression of inflammatory mediators COX-1, IL-1β and IL-6 and mRNA expression of anti-inflammatory cytokine IL-4 in ankle articular tissues of right hind leg were represented in figures 4, 5, 6 and 7. The mRNA expression of COX-1 showed a significant increase in CFA-induced arthritic rats after 9 days while it exhibited a non-significant change after 18 days in comparison with normal control rats (Figure 4). On the other hand, the IL-1β and IL-6 mRNA expressions were significantly increased in CFA-induced arthritic rats after 9 and 18 days while IL-4 mRNA expression was significantly decreased (Figure 4-7). The administration of C. limon and C. paradisi fruit peel hydroethanolic extracts to arthritic rats significantly improved the altered COX-2, IL-1β, IL-6 and IL-4mRNA expressions.
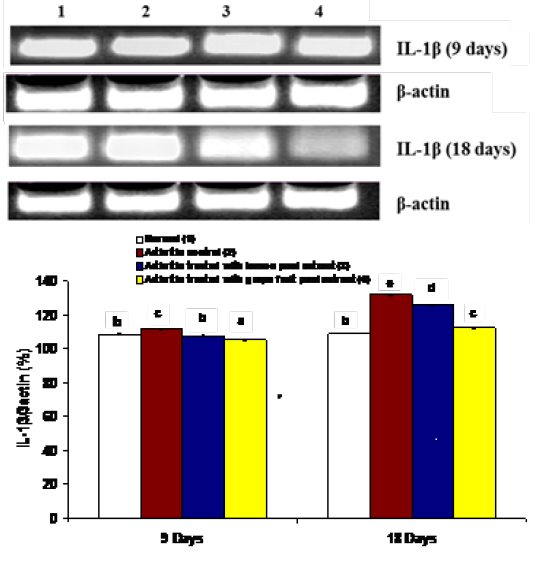
Figure 5: Effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on right hind leg ankle joint IL-1β expression relative to β-actin in CFA-induced arthritic rats. Means, which share the same superscript symbol(s), are not significantly different. Top: Gel photograph depicting IL-1β and β-actin PCR products Bottom: Corresponding intensity analysis of right ankle joint IL-1β PCR products from 3 different experiments, represented as the IL-1β mRNA percentage of β-actin mRNA.
The histological changes of the articular ankle joint in various groups were depicted in figure 8. In normal rats, the bone surfaces in the synovium are covered by an articular cartilage that lacks a perichondrium. The heads of the two articulated bones are enclosed and united by an articular capsule that consists of two parts, the outer and inner parts. The outer part is a sheath of fibrous tissue (fibrous capsule)

Figure 6: Effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on right hind leg ankle joint IL-6 expression relative to β-actin in CFA-induced arthritic rats. Means, which share the same superscript symbol(s), are not significantly different. Top: Gel photograph depicting IL-6 and β-actin PCR products Bottom: Corresponding intensity analysis of right ankle joint IL-6 PCR products from 3 different experiments, represented as the IL-6 mRNA percentage of β-actin mRNA.
which extends well beyond the articular cartilage of each bone. The inner part is nameda synovial membrane; it lines the fibrous capsule and is reflected onto the bone that covers right up to the articular cartilage. Thus, the joint cavity between two articulated bones is lined everywhere with either synovial membrane or articular cartilage. The synovial membrane is a thin sheath of fibrous connective tissue, with dense network of blood and lymph capillaries. Ankle joint sections (Figures 8-8 and 8-9) of rat from normal group after 9 and 18 days showed normal histological structure of an ankle with normal articulating cartilage, synovial cavity, sponge bone and bone marrow.
The characteristics of CFA-induced arthritic joints in rats are synovial hyperplasia, excessive proliferation of the synovium leading to the formation of pannus, the exudation of cells into the joint cavity and destructive changes and erosion of bone and cartilage. CFA-administered arthritis rats after 9 days showed necrosis of cartilage with inflammatory cells infiltration and fibroblasts proliferation in an ankle joint sections (Figure 8-10a) and degeneration of cartilage,
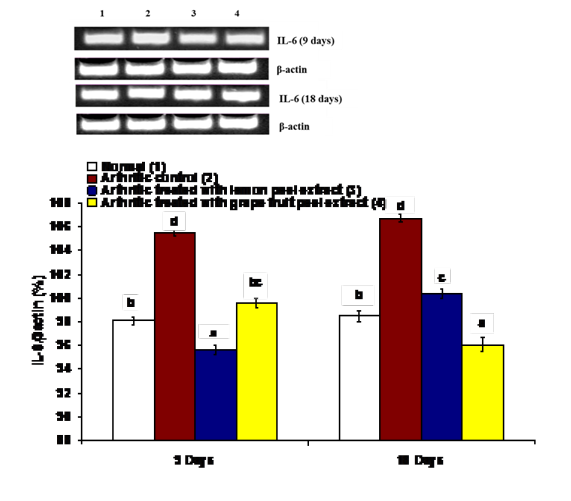
Figure 7: Effect of C. limon and C. paradisi fruit peel hydroethanolic extracts on right hind leg ankle joint IL-4 expression relative to β-actin in CFA-induced arthritic rats. Means, which share the same superscript symbol(s), are not significantly different. Top: Gel photograph depicting IL-4 and β-actin PCR products Bottom: Corresponding intensity analysis of right ankle joint IL-4 PCR products from 3 different experiments, represented as the IL-4 mRNA percentage of β-actin mRNA.
inflammatory cells infiltration and pannus formation (Figure 8-10b) while arthritic rats after 18 days revealed necrosis of cartilage and inflammatory cells infiltration (Figure 8-11a), marked oedema (Figure 8-11b), massive inflammatory cells infiltration and pannus formation (Figure 8-11c) and proliferation of synovial membrane, necrosis of cartilage and fibroblasts proliferation(Figure 8-11d).
CFA-administered rats treated with C. limon hydroethanolic extract showed no histopathological changes in right hind ankle joint sections after 9 days (Figure 8-12) and 18 days (Figure 8-13). On the other hand, the treatment of CFA-induced arthritis with C. paradisi hydroethanolic extract showed inflammatory cells infiltration and pannus formation (Figure 8-14a), proliferation of synovial membrane and necrosis of cartilage (Figure 8-14b) in ankle joint sections after 9 days. As the period of treatment with C. paradisi hydroethanolic extract extended to 18 days, right hind ankle joint section revealed inflammatory cells infiltration (Figure8-15).

Figure 8: Photomicrograph of a section in ankle joint of rat; 8: normal group after 9 days, 9: normal group after 18 days, 10: arthritic control group after 9 days, 11a-c: arthritic control group after 18 days, 12: arthritic group treated with C. limon fruit peel after 9 days, 13: arthritic group treated with C. limon fruit peel after 18 days, 14 a-b: arthritic group treated with C. paradisi fruit peel after 9 days, 15: arthritic group treated with C. paradisi fruit peel after 18 days ( H & E X 100).
The spleen section of normal group exhibited normal histological structure of the lymphoid cells in white pulps as well as the histological structure of the red pulps after 9 days (Figure 9-16) and after 18 days (Figure 9-17). CFA-induced arthritic rats after 9 days showed slight lymphocytic necrosis and massive hemorrhage in spleen sections (Figure 9-18a and 9-18b) and mitotic figure appearance (Figure 9-18c). Meanwhile, spleen sections of arthritic control group after 18 days showed mitotic figure appearance (Figure 9-19a), thrombus formation (Figure 9-19b) and hemorrhage (Figure 9-19c). CFA-induced arthritic rats supplemented with C. limon hydroethanolic extract showed no histopathological alteration in spleen sections after 9 days (Figure 9-20) and 18 days (Figure 9-21). The same for CFA-induced arthritic rats treated with C. paradisi hydroethanolic extract which revealed no histopathological alteration in spleen sections after 9 days (Figure 9-22) and 18 days (Figure 9-23).
The thymus of normal rats after 9 and 18 days depicted normal histological structure of lymphoid cells in both peripheral cortex and central medulla with no histopathological changes (Figures 9-24a& b and Figures 9-25a& b).Thymus sections from arthritic control rats after 9 days showed lymphoblasts’ activation and mitotic figure (Figure 9-26). After 18 days, thymus of arthritic rats revealed lymphoblasts activation and mitotic figure (Figure 9-27a), lymphoblasts activation and congested blood capillaries (Figure 9-27b).The arthritic group treated with C. limon fruit peel hydroethanolic extract for 9 and 18 days showed improved histological structure to near normal histological appearance indicating no histopathological changes in thymus sections after 9 and 18 days (Figure 9-28 and 9-29).Section in thymus of rats from arthritic group treated with C. paradisi fruit peel hydroethanolic extract after 9 and 18 days revealed no histopathological changes as shown in figure 9-30 and 9-31 for 9 and 18 days respectively.

Figure 9: Photomicrograph of; 16: section in spleen of rat from normal group after 9 days, 17: section in spleen of rat from normal group after 18 days, 18 a-c: sections in spleens of rats from arthritic control group after 9 days, 19 a-c: sections in spleens of rats from arthritic control group after 18 days, 20: spleen of rat from arthritic group treated with C. limon fruit after 9 days, 21: spleen of rat from arthritic group treated with C. limon peels after 18 days, 22: spleen of rat from arthritic group treated with C. paradisi peels after 9 days,
DISCUSSION
RA is considered as one of the most common chronic inflammatory autoimmune diseases. It is distinguished bysystemic inflammation, permanent synovitis, edema and production of auto antibodies (Scott and Wolfe, 2010). RA is chronic progressive and it results in significant morbidity and premature mortality (Snekhalatha et al., 2013). Prevalence of RA varies between 0.3% and 1% worldwide and is more common in developed countries than in developing countries (Snekhalatha et al., 2013). In India, the predominance of RA was recorded to be 0.75% in the adult population (Mathew et al., 2009). Although RA is an advanced inflammatory joint disease, which affects about 1% of the population worldwide, its etiology remains to be clearly demonstrated (Mattey et al., 2007; Karmakar et al., 2010; Bax et al., 2011). AIA is one of the most characterized animal models of RA that provides substantial insights into basic pathogenic mechanisms and imposes potential novel drugs for the therapy of human RA. CFA is commonly used to induce experimental model of arthritis and is considered to be the most effective available adjuvant. This model mimics much of the pathology of human RA including hyperplasia of the synovial tissues, inflammatory infiltration of the joints and synovitis as well as destruction of articular bone and cartilage (Chou et al., 2011). In the present study, it was found that rats injected with CFA for 9 and 18 days induced significant increase in paw swelling and edema manifested by increased right hindpaw circumference, volume and thickness. These increases in arthritic indexes reflect the swelling rate due to inflammation and edema, thickening of synovial membrane, redness of paw and ankle regions and congestion in blood vessels which are evidenced by histopathological findings.
In the present study, the treatments of CFA-induced arthritic rats with C. limon and C. paradisi fruit peel hydroethanolic extracts at the dose level 100 mg/kg b.w. induced a significant decrease in markers of paw swelling and edema including circumference, volume and thickness of right hind paw. The result of the present study isin conduction with the previous publications which indicated that there is a close relationship between the extent of inflammation and arthritic index (Rovenský et al., 2009). In support of this elucidation, Rasool and his colleagues reported that increased paw swelling in the arthritic mice was explained to be a result of edema of periarticular tissues (Rasool et al., 2006).The decrease in paw circumference, volume and thickness as a result of C. limon and C. paradisi fruit peel hydroethanolic extracts treatment was associated with appearance of mild inflammatory cells infiltration after 9 days and 18 days in the current histological findings. As depicted in the present histological observations, the ankle joint of CFA-induced arthritic rats exhibited congestion and dilation of synovial blood vessels, necrosis of articular cartilage and inflammatory cells infiltration after 9 and 18 days. These histological deteriorations may be attributed to the fact that the articular and peri-articular inflammatory cells may act as mother cells for osteoclasts/chondroclasts, in cartilage and bone; osteoclasts/chondroclasts may play a substantial role in the erosion and destruction of cartilage and bone in arthritis (Ahmed et al., 2015).
It is worth mentioning that peripheral blood might not be the best indicator of disease progression in RA and does not necessarily correlate well with the clinical response. Therefore, this study investigated the effects on mRNA gene expression for the biomarkers COX-1, IL-1β, IL-6 as pro-inflammatory and inflammatory cytokines and IL-4 as anti-inflammatory cytokine at the site of disease, the ankle joint. In conduction with the data of the present study, the pro-inflammatory and inflammatory cytokines, which are exacerbated in human RA synovium, have also been shown to contribute to the development and pathophysiology of arthritis in the experimental animal models of RA (Marinova‐Mutafchieva et al., 1997; Schrier et al., 1998; Rioja et al., 2004).
Cytokines are substantially involved in the pathogenesis of a broad diversity of inflammatory disorders and autoimmune diseases. Cyclooxygenase (COX) is a key rate-limiting enzyme for prostaglandins (PG) output (Chen et al., 2014). This enzyme catalyzes the reaction in which arachidonic acid is converted into prostaglandin H2 (PGH2); the latter is further bio-transformed into different types of prostaglandin S (PGS) (Yar et al., 2011). These PGS are implicated in many human physiology and pathophysiological processes. Prostaglandin E2 (PGE2) is a vital inflammatory-disease mediator, which is significant in inflammatory processes, including fervescence and changes of vascular endothelial permeability with consequent edematous syndromes (Kundu et al., 2006; Shindler et al., 2010; Yar et al., 2011). Traditional NSAIDs display anti-inflammatory effects and make side effects that are correlated with COX. Two COX isoforms have been distinguished into COX-1 and COX-2 which triggers PGE2 production and predisposes inflammatory disorders (Shindler et al., 2010).
Output of cytokines and chemokines in RA is induced by macrophage-, fibroblast-derived and lymphocyte-derived cytokines (Furst and Emery, 2014). Of these, proinflammatory cytokines, IL-1β and TNF-α are often targeted in RA treatment designs (Bresnihan et al., 1998; Zhuang et al., 2013). Regarding proinflammatory cytokines, CFA-induced arthritic rats treated with C. limon and C. paradisi fruit peel hydroethanolic extracts displayed significant decrease in TNF-α, IL-1β, and IL-6 when compared to the CFA-control group. RA is initiated by a T cell-mediated immune response that catalyzes the liberation of cytokines and promotes formation of antibody, which leads to damage of the joint (Porth, 2011). Particularly, TNF-α promotes the production of IL-1β and IL-6 (Brennan and McInnes, 2008), with the former being a substantial mediator of the inflammatory response, leading to various auto-inflammatory syndromes (Masters et al., 2009; Kim et al., 2016), and the latter being secreted by macrophages and T cells to initiate and stimulate the immune response (van der Poll et al., 1997; Febbraio and Pedersen, 2005). So, proinflammatory cytokines particularly IL-1β, IL-6, and TNF-α play an important role in arthritis starter(Choy and Panayi, 2001), while inhibitors of these cytokines are influenced in controlling chronic inflammation (Tracey, 2002; Pavlov and Tracey, 2006).
However, as a typical anti-inflammatory cytokine, IL-4 was extremely down-regulated in its mRNA expression in the arthritic animals. This is in line with previous literatures which demonstrated that IL-4 mRNA was not detected in spleen using competitive RT-PCR throughout the course of AIA (Pohlers et al., 2005). This finding suggests that the injection of CFA to the albino rats enhances the inflammatory mediators while suppresses the anti-inflammatory mediators, thus develops the inflammation process. IL-4 can therefore act as an anti-inflammatory interleukin in autoimmune diseases as evidenced by its potent protective effect in murine models of (Morita et al., 1998; Woods et al., 2001). Thus, it could be considered as a target for the treatment of these diseases. IL-4 was chosen as a therapeutic agent based on its established anti-inflammatory activities and its relative absence from the rheumatoid joint (Boyle et al., 1999). Addition of exogenous IL-4 to cultures of RA synovial tissue cells or synovial tissue explants suppresses synthesis of pro-inflammatory cytokines such as IL-1, IL-6, TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) as well as metal loproteinases (Lacraz et al., 1995; Nemoto et al., 1997).
Histopathologically, the ankle joint of CFA-administered arthritic rats exhibited deleterious histological changes including synovial hyperplasia, eroded articulating cartilage and pannus formation (Tag et al., 2014). These histopathological perturbations may be attributed to the increase in the oxidative stress, suppression of antioxidant defense system, elevation in the pro-inflammatory and inflammatory cytokines represented by increased IL-1β, IL-6 and COX-1 mRNA expression and depletion of the anti-inflammatory cytokines represented by decreased IL-4 mRNA expression after 9 and 18 days. The improvement of ankle joint histological architecture as a result of treatment of arthritic rats with C. limon and C. paradisi fruit peel hydroethanolic extracts may be, in turn, due to the ability of these extracts to scavenge lipid peroxides and free radicals, enhance the antioxidant defense system and suppress inflammatory status.
The spleen, a major organ of the body’s immune system, filters out and destroys old erythrocytes, removes antigens by phagocytosis and plays a key role in prevention against infection (Handy et al., 2002). In the present study, the spleen sections of normal rats exhibited normal histological structure of the lymphoid cells in white pulps as well as the histological structure of the red pulps. The Photomicrographs of the arthritic control group showed lymphoid hyperplasia in the white pulps. This finding is in line with the previous studies which demonstrated that during the course of arthritis in the rat, splenomegaly is a prominent feature due tolymphoid hyperplasia, splenitis and abscess formation (Issekutz and Sapru, 2008).
Thymus is also a major organ of the body’s immune system that consists of peripheral cortex (primarily composed of lymphocytes or thymocytes, with a few epithelial cells and mesenchymal cells) and central medulla which is composed of fewer lymphocytes but more epithelial cells. It normally processes immature precursor T-lymphocytes (derived from bone marrow and initially located in the outer cortex of the thymus) into the mature immunocompetent T-cells of the medulla (Vos and Van Loveren, 1998). In the current study, the thymus sections of normal rats depicted a normal histological structure of the lymphoid cells in both cortex and medulla. Thymus sections from arthritic control rats showed widening (hyperplasia) of the medulla with minimal necrobiotic changes and congestion of the blood vessels and mononuclear cell infiltration in the area surrounding blood vessels. These results are in accordance with the previous studies reported that lymphoid hyperplasia (increased number of lymphoid follicles) of the thymus is observed in a number of immunologically mediated disorders, including RA (Nishino et al., 2006).
The treatment with oral administration C. limon and C. paradisi fruit peel hydroethanolic extracts, at oraldose 100 mg/kg b. wt, potentially ameliorated and reduced inflammation and joint destruction with absence of most histological lesions depicted in CFA-induced arthritic rats associated with no histopathological changes of spleen and thymus sections. These improvements may be secondary to suppressions of oxidative stress and inflammation in addition to the enhancement of antioxidant defense system after 9 and 18 days.
CONCLUSION
The present results demonstrated that C. limon and C. paradisi fruit peel hydroethanolic extracts effectively suppressed the pathological development CFA-induced arthritis and possessed a potential anti-arthritic, anti-inflammatory efficacies in CFA-induced arthritic rats. Thus, the treatment with C. limon and C. paradisi fruit peel hydroethanolic extracts may have potential therapeutic effects in the remedy of RA. However, further studies are required to assess the efficacy of C. limon and C. paradisi fruit peel hydroethanolic extracts in arthritic human beings.
ACKNOWLEDGEMENTS
The authors thank Prof. Dr. Ahmed KA, Professor of Pathology, Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt and Prof. Dr. Ahmed RR, Professor of Molecular Cell Biology, Division of Cell Biology, Histology and Genetics, Department of Zoology, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt for helping in examining and reading the histological stained sections.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Authors Contribution
Osama Mohamed Ahmed proposed the research plan, guided the experimental work and shared in writing the manuscript. Mohamed Badr Ashour revised the manuscript and also supervised the experimental work. Hanaa Ibrahim Fahim participated in writing and revising the manuscript. Noha Abdelazeem Ahmed performed the experimental work and participated in writing the manuscript.
REFERENCES





