Advances in Animal and Veterinary Sciences
Review Article
Assessment of Mucosal and Systemic Immune Responses against Staphylococcus aureus in Ruminants
Lawan Adamu1, 6,*, Jesse Faez Firdaus Abdullah1, 2, Bala Jamilu Abubakar4, 10 ,Idris Umar Hambali1,5, Odhah Mohammed Naji1, 3, Arsalan Maqbool7, 8, Muhammad Naveed Ali7, Bhutto Khaleeq ur Rehman1, 9, Abraham Gabriel Abdullah1, 2, Wahid Abd Haron1, Mohd Azmi Mohd Lila4, Mohd Zamri Saad4
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; 2Department of Farm & Exotic Animals Medicine & Surgery, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM), 43400 UPM, Serdang, Selangor, Malaysia; 3Department of Veterinary Medicine, Faculty of Agriculture and Veterinary Medicine, Thamar University, Yemen; 4Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia; 5Department of Veterinary Public Health and Preventive Medicine, University of Maiduguri, Nigeria; 6Department of Veterinary Medicine, University of Maiduguri, Nigeria; 7Institute of Tropical Agriculture and food security, Universiti Putra Malaysia; 8Livestock and Dairy development Department Baluchistan, Pakistan; 9Veterinary Research & Diagnosis, Livestock and Fisheries Department Sindh, Pakistan; 10Department of Medical Laboratory Science, Faculty of Allied Health Sciences, Bayero University Kano, P. M. B. 3011, Kano, Nigeria.
Abstract | Staphylococcus aureus is the most important human and animal pathogen responsible for a wide spectrum of morbidity and acute clinical infections, in addition to tenacious chronic forms of diseases. The pathogen sophisticated virulence, and its abilities to abate or elude the host immune responses by the myriad of processes makes it the most dreaded organisms, both in the communities, hospital setups and the dairy industries worldwide. S. aureus vaccines have revealed a significant challenge because of plentiful virulence physiognomies. For these reasons, numerous protein particles and several potent transporters of these proteins called adjuvants were proposed as ideal vaccines contrivances for the prevention of Staphylococcus aureus infections. Furthermore, for the formulation of these vaccine contraptions nascent technologies which include the Bioinformatics, Proteomics, Metagenomics, Metabolomics, Transcriptomics and Nanotechnology and its ability in the deliverance of vaccines in research are similarly advocated, an intact procedure employed for the evaluation of the vast proteins and genes that were disclosed by a microorganism is currently available. Likewise, existing are marvellous expectation in these burgeoning expertise in understanding the microbial and host affiliations. In view of this, the blossoming facts may perhaps tremendously assure to the headway of immunogenic vaccines as anti-Staphylococcus aureus contagions in both hominids and animals. This periodical highlights and explicates on the up-to-date eminence of mucosal and systemic immune responses by the application of Transcriptomics, Metabolomics, Metagenomics, Proteomics and Nanotechnology techniques for their prominence in the evaluation of refined proteins for use as systemic and mucosal immunogenic vaccines for forefending of Staphylococcus aureus contagions in goats, sheep and cattle.
Keywords: Mucosal, Systemic, Immune responses, Staphylococcus aureus, Ruminants
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 05, 2018; Accepted | August 12, 2018; Published | September 21, 2018
*Correspondence | Lawan Adamu, Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; Email: drlawan3758@yahoo.com
Citation | Adamu L, Abdullah JFF, Abubakar BJ, Hambali IU, Naji OM, Maqbool A, Ali MN, Rehman BK, Abdullah AG, Haron WA, Lila MAM, Saad MZ (2018). Assessment of mucosal and systemic immune responses against staphylococcus aureus in ruminants. Adv. Anim. Vet. Sci. 6(10): 413-426.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.10.413.426
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Adamu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Staphylococcus aureus is a Gram positive bacterium and the most imperative animal and human pathogen responsible for a wide spectrum of morbidity and acute clinical infections, in addition to persistent chronic forms of diseases, like bacteremia, endocarditis, osteomyelitis, skin and soft tissue infections including mastitis in dairy cows and associated with enormous economic losses second to loses encountered in Orf virus infection in small ruminants (Zamri-Saad et al., 1999; Joshi and McNeely, 2013; Azhar et al., 2016; CoÃteÂ-Gravel et al., 2016). Amongst factors that can elucidate the botch of antibiotherapy and the proclivity to cause chronic infections, which could be ascribed to the pathogen’s multidimensional virulence, largely to its abilities to weaken or evade the host immune responses by toxin secretion (Park et al., 2011; CoÃteÂ-Gravel et al., 2016), establishment of biofilm (Rice et al., 2007; Otto, 2013; CoÃteÂ-Gravel et al., 2016) and its persistence in nonphagocytic host cells, which could safeguard the pathogen from the attack of the host immune system and antibiotics (Brouillette et al., 2004; CoÃteÂ-Gravel et al., 2016). Additionally, commonness of S. aureus infections are becoming more bothersome with the development of compound antibiotic resistant strains (Chambers and Deleo, 2009; García-Álvarez et al., 2011; CoÃteÂ-Gravel et al., 2016).
S. aureus vaccines have divulged a substantial challenge because of numerous virulence features. An important virulence feature seems to be the capsular polysaccharide (CPS) of field strain which was divulged by S. aureus when grown on whey augmented medium (Hong-Ryul and Hee-Myung, 2000). Consequently, it seems that the vaccine that has the ability of conferring fortification from staphylococcal infections is required to encompass CPS. Likewise, an elevated concentration of circulating alpha toxin antibody were also indispensable for defence from extremely toxigenic strains. CPS antibody in ruminants should be principally IgG2 since neutrophils possesses an Fc receptor merely for this particular immunoglobulin isotype. Similarly, deactivation of bacteriological toxin can be mediated by both IgG2 and IgG1 antibody (Hong-Ryul and Hee-Myung, 2000). The significant complications in the formulation of the vaccine is this, it requires a sizeable and long-lasting IgG2 anti-CPS response in addition to a robust and incessant upsurge of circulating levels of alpha toxin antibody which is indispensable. Furthermore, it has been demonstrated that it was tremendously challenging to accomplish IgG2 anti-S. aureus responses with killed vaccines (Hong-Ryul and Hee-Myung, 2000).
Several virulence features were described in S. aureus from the mastitis of cows, these include leukocidin, exfoliative toxins; ETA to ETD, haemolysins; HLA and HLB, staphylococcal enterotoxins (SEs), toxic-shock syndrome toxin-1 (TSST-1), and biofilm formation (Rice et al., 2007; Zimmer et al., 2006; Haveri et al., 2008; Jia et al., 2015). Excepting their pathogenic significance in mastitis of cows, S. aureus strains that produce toxin are greater risk for animals and humans (Francis et al., 2005; Jia et al., 2015). The toxin genes are largely positioned on the genetic elements that are mobile, and consequently can be transmitted amongst staphylococcal species or isolates (Lawrynowicz-Paciorek et al., 2007; Vasconcelos and da Cunha, 2010; Jia et al., 2015). Even though these toxin genes were initially described in S. aureus isolates, while others were identified in multiple coagulase-negative staphylococci (CNS) species from the udder of other ruminants especially cattle (Park et al., 2011; Jia et al., 2015), which might conceivably be a reservoir of toxin genes classically recognized in S. aureus. Nevertheless, merely few research have thus far been engrossed in the virulence features of CNS identified from the mastitis of cow (Vanderhaeghen et al., 2014; Jia et al., 2015).
Numerous clinical isolates of S. aureus are encapsulated, and serotype 5 and 8 strains predominate (Andrew et al., 2005). The type 5 (CP5) and type 8 (CP8) capsular polysaccharides have analogous trisaccharide iterating units consisting of N-acetyl D-fucosamine, N-acetyl L-fucosamine and N-acetyl mannosaminuronic acid (Andrew et al., 2005). CP5 and CP8 are serologically dissimilar, and this can be ascribed to the modifications in the bonds between the sites of O acetylation and the sugars. Accordingly, there is an exigent need to find potential new strategies to control Staphylococcus aureus.
The mucous membranes coating the digestive and the urogenital tracts in addition to the innermost part of the ear, the eye conjunctiva and the ducts of all exocrine glands are able to act as a potent chemical and mechanical cleansing mechanisms that destroy and fend off most extraneous substances. Furthermore, a huge and extremely well equipped innate and adaptive mucosal immune system safeguard these surfaces, and including the innermost part of the body, against impending attacks from the surroundings. In a healthy animal, virtually 80% of all the immunocytes is contributed by the immune system. These cells are predominantly found traversing between, the numerous mucosa-associated lymphoid tissues (MALT), which collectively form the major animal lymphoid organ system (Ogra et al., 2012).
Three foremost functions attributable to the mucosal immune system include protection of the mucous membranes against colonization and incursion by potentially perilous microorganisms, to avert the uptake of viable antigens comprising of alien proteins obtained from consuming food products, commensal microorganisms and airborne particles and also to avert the progression of potentially injurious immune responses to the antigens on reaching the innermost part of the body (Jan and Cecil, 2005). The dissimilarity with the systemic immune contrivance, which performs optimally in a typical microbial free environment and repeatedly reacts robustly to intruders, the MALT frequently safeguard tissues that are overwhelmed by foreign substances. On encountering massive antigenic stimulation, the MALT parsimoniously choice apposite effector mechanisms to control the strength of the invasion in order to evade tissue injury and immunological lassitude (Jan and Cecil, 2005).
Other than the characterization of significant virulence factors of S. aureus, the utilization of an adjuvant that can stimulate potent and protracted immune response is imperative to the progression of a prosperous staphylococcal vaccine (Hong-Ryul and Hee-Myung, 2000; Zecconi et al., 2006). Some constituents, designated as adjuvants, have been incorporated with vaccines in an effort to make them more potent. In the progression of new-fangled vaccines, there is an aspiration of simplifying immunization programs, both by reducing the amount of doses essential to acquire fortification and by escalating the quantity of antigens of each vaccine. In order to accomplish this, new-fangled and more potent adjuvants are prerequisite (Tollersrud et al., 2002).
The physiognomies of quite a lot of vaccine adjuvant formulations that possess the capacity of prompting mucosal and systemic immune responses divulged that dendritic cells of the skin could perhaps act as effective antigen presenting cells (APC) for mucosal and systemic immune responses, if the settings of the microbial environment is pertinently controlled following immunostimulation. Effective adjuvants of mucosal and systemic domains, in addition to microbiological toxins, biochemical boosters of vitamin D3 and cAMP, all possess similar abilities in prompting dendritic cell repositioning from the skin to Peyer’s patches following immunostimulation (Jennifer et al., 2009).
For the justification of current development in transcriptomics, metabolomics, metagenomics, and proteomics exploration, an all-inclusive appraisal of the immense genes and proteins that were made known by a microorganism is currently manageable. Besides, existing is a spectacular likelihood of progression in this expanding expertise in understanding the bacteria and host relationship. For this reason, the prosperous facts may perhaps tremendously assure the development of immunogenic vaccines against Staphylococcus aureus contagions in both hominids and animals. Therefore, this periodical highlights and explicates on the up-to-date eminence of mucosal and systemic immune responses by the application of Transcriptomics, Metabolomics, Metagenomics, proteomics and Nanotechnology techniques for their prominence in the evaluation of refined proteins for use as systemic and mucosal immunogenic vaccines for forefending Staphylococcus aureus contagions in goats, sheep and cattle.
Adjuvants For The Delivery Of Staphylococcus Aureus Vaccine Candidates
Adjuvants could perchance be imperative to upsurge IgG secretion in immunized faunas as a basis of antibodies in the dynamic immunity of ruminant’s diseases. Consequently, there has been an incessant exertion to seek for the additional successful vaccine adjuvants in ruminants.
The antibody concentrations of alpha toxin, of four adjuvant used, dextran sulfate (DXS), Freund’s complete adjuvant (FCA), immune stimulating complex (ISCOM) and aluminium hydroxide (Hydrogel), DXS adjuvant expressed the maximum antibody titer at 56 days post vaccination (Hong-Ryul and Hee-Myung, 2000). Whereas, in the agglutination titer against CPS, ISCOM and Hydrogel adjuvant expressed the maximum agglutination titer at 56 days post vaccination (Hong-Ryul and Hee-Myung, 2000). The use of DXS and aluminium hydroxide or mineral oil adjuvants if applied parsimoniously the immune response may express auspicious effects. Therefore, this feasibility divulged that when numerous antigens are utilized in vaccine of ruminant and combination of adjuvants may be more potent and beneficial than a single adjuvant in immune response. Additional mineral oil emulsions, such as Marcol, Drakeol, ISA-25 and ISA-206, are also utilised in numerous cattle vaccines (Enioutina et al., 2000; Singh and O’Hagan, 2003). In recent times, MF59, a discrepancy of the recyclable oil Squalene, has been verified to be an effective adjuvant with an acceptable protection record and consequently, is apposite for use in animals (Singh and O’Hagan, 2003; Jennifer et al., 2009).
Dexamethasone could perchance be used as Adjuvant for Staphylococcus aureus infections vaccine candidate, this is perhaps due to the amassed data portraying the enormous attributes and the beneficial outcome of dexamethasone to its prospective ability of reducing inflammatory mediators (Barnes, 2006). By impeding numerous inflammatory pathways, dexamethasone assuages the pathogenic immunological interplay between the invader and the host and leads to minimal tissue mutilation. Additionally, in the inflammatory setting, leukocytes are routinely assumed to be major contributors (Ioannis et al., 2007). There were numerous in vitro studies which also backed the finding that dexamethasone influences phagocytosis by animal monocytes, not predominantly of S. aureus, but of other macromolecules also, and in so doing could perchance contribute to the tissue reparation after immune-mediated tissue mutilation or infection (Veltrop et al., 2000; van der Goes et al., 2000; Ioannis et al., 2007).
A study was conducted to assess the relative efficacy of diterpene alcohol, phytol and its hydrogenated derivative PHIS-01, comparative to incomplete Freund’s adjuvant (IFA), a frequently used adjuvant in enhancing defensive immunity in mice against S. aureus, in terms of inflammatory cytokines (So-Yon et al., 2006). The outcome of their research divulged that vaccine preparations encompassing phytol and PHIS-01 as adjuvants convene a strong and shielding immunity against both Gram-positive and Gram-negative microorganisms without stimulating an adversative inflammatory cytokine as a result of IL-6 (So-Yon et al., 2006).
While the closely interrelated E. coli heat-labile enterotoxin (LT) and cholera toxin (CT) act as potent mucosal adjuvants when administered together with solvable antigens. Both LT and CT comprise of a homopentamer of cell-binding B subunits related with a lone toxic active A subunit. In the affected cells there is an elevated secretion of cAMP which is cause by the A subunit that enzymatically ribosylates the GS protein of adenylate cyclase. In recent times, mutagenesis has allowed the expression of CT and LT mutants that have abridged toxicity, but which preserve momentous adjuvanticity when administered to animals via the nasal-mucosal itinerary or, albeit they perform poorly, by the oral-mucosal itinerary (Pizza et al., 2001; Kristina and Jan, 2002). An alternative method that is employed is by evading the injurious downsides of LT or CT adjuvants is by connecting the enzymatically active A subunit part of the toxin to a cell attachment moiety excluding the normal B subunit, for instance the cell attachment part of Staphylococcus aureus protein A (CTA1–DD). CTA1–DD, similar to some toxic by-products, are efficacious nasally, but not orally (Jan and Cecil, 2005). This difficulty has in recent times been resolved by the combination of CTA1–DD and immune stimulating complexes (ISCOMS). These combinations are equal colloidal particles comprising of the saponin-adjuvant Quil A, phospholipids, cholesterol and a chosen antigenic protein (Kristina and Jan, 2002). Oral immunization with the ISCOM–CTA1–DD complex stimulated mucosal and systemic responses with both Th1 and Th2 physiognomies (Mowat et al., 2001; Jan and Cecil, 2005). Conversely, many vaccinations were essential, indicating that the process requires additional enhancements.
To detoxify CT, additional mutants were demonstrated whereby peptides were incorporated into the terminal of CTA1 amino acid. The incorporated peptides appear to diminish both ADP-ribosylating and enterotoxicity performance using sterical prevention by means of the CTA1 dynamic position (Jan and Cecil, 2005). On the whole, like other detoxified concepts, the ability of an adjuvant to retain its potency diminished with diminishing enterotoxicity or ADP-ribosylation (Jan and Cecil, 2005). Nevertheless, eCT6 is another mutant, which has a much inferior enterotoxicity, exhibited a superior adjuvanticity analogous to the CT wild type. An additional mutant with an extensive peptide connected to CTA1 (eCT23) and without any obvious toxic effect, even though is much less effective in adjuvanticity than either eCT6 or CT, was better than CTB as both adjuvant and mucosal immunogen for a combined inoculated antigen (Jan and Cecil, 2005).
Microbial DNA encompassing of cytosine, phosphate and guanosine (CpG) complex, wherein the cytosine is unmethylated, in addition to synthetic oligodeoxynucleotides comprising of immunostimulatory CpG complex (CpG ODN), have well-typified adjuvant features when inoculated systemically coupled with an antigen (Krieg et al., 2002; Jan et al., 2003). The potent physiognomies of CpG DNA as an adjuvant, as a result of the attachment of toll-like receptor-9 (TLR-9) to CpG-rich DNA, is related to the stimulation of both pro-inflammatory and Th1-stimulating chemokines and cytokines, and the stimulation of major histocompatibility complex (MHC) and other induction molecules on APC. The resulting immune responses in mice were dominated by Th1 with escalated concentrations of IgG2a, IFN-γ and cytotoxic T lymphocytes (Krieg, 2002; Jan et al., 2003).
Some topical studies also divulged that CpG ODN as mucosal adjuvants are similarly efficacious. Inoculation of CpG ODN intranasally coupled with refined protein antigens enhances a systemic Th1 response and a mucosal Th2 response with IgA antibody development (McCluskie et al., 2000; Jan and Cecil, 2005). The associated emergence of mucosal IgA antibodies that can avert the phagocytosis of mucosally conveyed microorganisms in combination with systemic complement-stimulating antibodies and cytotoxic T cells should be of enormous advantage in opposing numerous infections departing from a mucosal surface (Zamri-Saad et al., 1999; McCluskie et al., 2000; Jan and Cecil, 2005).
Accordingly, a lone vaginal mucosal inoculation of CpG ODN stimulated speedy generation of the Th1 related cytokines (IL-12, IL-18 and IFN-γ) in addition to MIP-1α, MIP-1α and CC chemokines RANTES in the mucosa of the genital tract of a murine female (Jan and Cecil, 2005). Immunostimulatory of CpG DNA heightens innate immunity in the mucosa of the gastrointestinal tract. Hence, intragastric inoculation by means of CpG ODN has been indicated to stimulate local generation of MIP-1α, MIP-1α, CC chemokines RANTES and of CXC chemokine IP-10 in the gastrointestinal tract (Jan and Cecil, 2005).
Large number of adjuvants apply their adjuvant ability via the stimulation of inflammatory or Th1-stimulating chemokines and cytokines. As a general rule, the more effective the adjuvant, the more unsuitable it is for animal use (Jan and Cecil, 2005). The additional technique of side-stepping the difficulty with explicitly toxic adjuvants is to simulate the signs they stimulate in a living organism by merely incorporating these inciting molecules both indirect as coding DNA and directly as proteins. The most potent mucosal adjuvants known up to the present time are the LT and CT, which stimulate robust mucosal IgA responses, systemic IgG responses and CTL to combine inoculated antigens (Jan and Cecil, 2005). Numerous mixtures of cytokines have been divulged as proficient of substituting LT or CT as a suitable nasal adjuvant. The most significant is IL-1, which, when combined with Th1-stimulating cytokines for instance GM-CSF, IL-12 and IL-18, can elicit sturdy systemic and mucosal responses as CT (Staats et al., 2001; Jan and Cecil, 2005). The mixture of GM-CSF, IL-1, IL-12 and IL-18 produce an augmented Th1; IFN-γ and CTL or Th2 a mucosal IgA alongside feeble antigens for example the cloned peptides (Staats et al., 2001;Jan and Cecil, 2005).
The combination of genes coding for particular chemokines, for instance CCR7 ligands which are included in leading DC to the T-cell domains of the ancillary lymphoid organs, to a Herpes simplex virus type 2 (HSV-2) DNA plasmid vaccine has induced the immune responses following both nasal and intragastric immunization in mice (Eo et al., 2001; Jan and Cecil, 2005). The utilization of RANTES a chemoattractant for T cells, NK cells, monocytes and an effective stimulator of Th1 and CTL responses as a mucosal adjuvant has a tremendous auspicious preliminary outcomes. Nasal combined inoculation of RANTES and a protein antigen has been indicated to improve Th1 and Th2 response either at the local and or distant mucosal tissues in addition to systemic response (Lilliard et al., 2001; Jan and Cecil, 2005).
Vaccine Candidates against Staphylococcus Aureus
D-alanine auxotroph has the ability to prompt forefending immunity against staphylococcal contagion. Deletion of the mutant is extremely weakened and stimulates a defensive immune response in mice and produces cross-reactive antibodies. Furthermore, the D-alanine auxotroph was entirely removed from the blood of mice following intraperitoneal or intravenous inoculation. The defensive outcome has been reliant on antibody generation since the transmission of immune serum in naïve mice resulted in strong fortification against S. aureus. Moreover, splenocytes from mice vaccinated with the D-alanine auxotroph vaccine exhibited precise secretion of IL-17A after ex vivo induction. Thus, D-alanine auxotroph safeguards mice proficiently against virulent staphylococcal strains via the augmented performance of antibodies and IL-17A, and hence establishes an auspicious vaccine candidate against staphylococcal disease, for which no licensed vaccine has been obtainable yet (Adhikari et al., 2015; Miriam et al., 2018).
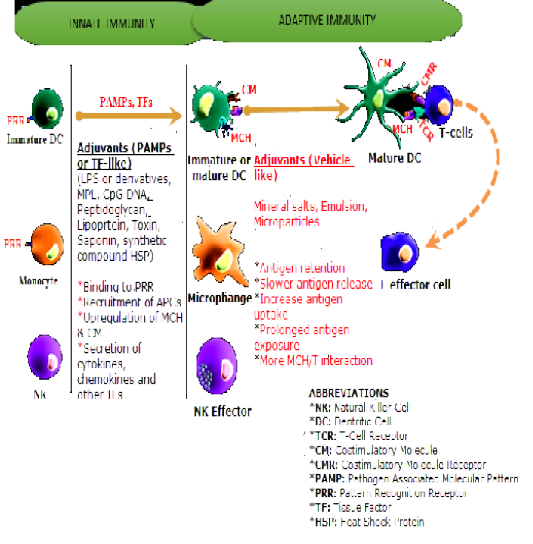
Figure 1: Influence of Adjuvants on Innate and Adaptive Immune Systems. Source Kensil et al., 2004, with some modifications.
A killed full cell lysate formulation (SaWCA) was used by lysing a USA 300 strain with lysostaphin using sonication by harvesting the supernatant portion. Vaccination using SaWCA and CT produced strong IL-17A but with comparatively mild antibody responses, and afforded fortification of the skin abscess, but not of the dermonecrosis or invasive infection classic (Fan et al., 2017). Compared with parenteral vaccination of SaWCA and alum generated strong antibody and IL-17A responses and safeguarded mice in all three prototypes. Sera produced after vaccination with SaWCA had quantifiable antibodies directed against six tested preserved surface proteins, and stimulated opsonophagocytosis ability against two S. aureus strains. Passive transfer of SaWCA-immune serum safeguarded mice against dermonecrosis and invasive infection, but afforded no obvious outcome against skin abscesses, proposing that antibodies alone may not be adequate for the fortification in this model. Consequently, vaccination with a SA lysate formulation produces strong antibody and T cell responses, and provides fortification in systemic and cutaneous staphylococcal infection models (Azmi and Field, 1993; Fan et al., 2017).
The Staphylococcus aureus biofilm evolution is related to numerous protracted illness that are really challenging to cure as a result of the refractory state of biofilms to be eliminated with antibiotics (Rice et al., 2007; Zimmer et al., 2006; Haveri et al., 2008; Jia et al., 2015). Consequently, there is an intense effort in order to avert establishment of S. aureus biofilms and the emergence of effective antibiofilm vaccines. For the duration of biofilm related illness, the initial crossing point concerning the host and microorganisms is the inherent generated extracellular medium, the prospect of extracellular proteins established in the biofilm medium is to stimulate defensive immune response against S. aureus infections (Carmen et al., 2013). By utilizing proteomics technique, the exoproteomes of exopolysaccharide and the protein biofilm generated by two clinical S. aureus strains were typified. Extraordinarily, the outcome indicated that autonomous state of the biofilm medium, a shared core protein is enclosed in both forms of the exoproteomes. Intradermal inoculation of an exoproteome stimulated a humoral immune response and provoked the generation of IL-17 and IL-10 in mice. Antibodies against exoproteome stimulated opsonophagocytosis and the destruction of S. aureus which considerably associated with the generation of IgM and IgG with opsonic proclivity. This phenomena is similar to the antibodies produced in sheep and goats during Orf infection (Bala et al., 2018; Jesse et al., 2018). Vaccination with the biofilm exoproteome considerably decreased the quantity of microbial cells in the interior of the biofilm and on the adjacent tissues. Additionally, vaccinated mice also indicated some degree of organ habitation by microbes secreted from the medium at the breaking phase of the biofilm sequence. Overall, the prospect of biofilm exoproteins as a propitious contender vaccine against S. aureus biofilm infections are intensely progressing (Carmen et al., 2013).
Numerous extracellular combinations were recognized as mediators of staphylococcal biofilms, for instance polysaccharide intercellular adhesin (PIA; also called poly-N-acetylglucosamine exopolysaccharide [PNAG]), (Vuong et al., 2004; Izano et al., 2008; Carmen et al., 2013), extracellular DNA (eDNA) [40, 42, 43], and dissimilar surface proteins, comprising of the fibronectin-binding proteins (FnBPs), SasG, biofilm-associated protein (Bap) and protein A (Cucarella et al., 2001; Corrigan et al., 2007; O’Neill et al., 2008; Merino et al., 2009; Vergara-Irigaray et al., 2009; Carmen et al., 2013). These biofilm mediators were suggested as vaccine antigens against S. aureus infections. Diverse researches revealed that inoculation of deacetylated PNAG mixed with diphtheria toxin as a transporter protein stimulates immunological response that safeguards against S. aureus infection (Maira-Litrán et al., 2002; Pérez et al., 2009; Carmen et al., 2013; Cywes-Bentley et al., 2013). In addition, a topical research (Cywes-Bentley et al., 2013) indicated that PNAG is a preserved superficial polysaccharide generated by several harmful microorganisms, protozoal parasites and fungi and established that inert vaccination with antibodies to PNAG safeguards mice against both systemic and local infections elicited by numerous harmful microorganisms (Carmen et al., 2013; Cywes-Bentley et al., 2013). FnBPs and Protein A were appraised for vaccine improvement. These antigens produce an immune response that offers incomplete fortification against S. aureus challenge using the model of infection systemically (Zhou et al., 2006; Kim et al., 2010; Kim et al., 2012; Carmen et al., 2013; Vinod et al., 2015). Conversely, no proof regarding effectiveness of these particles for the fortification against biofilm infections were acquired (Carmen et al., 2013).
Assessment of the genes coding for MSCRAMM (fib, fnbB, fnbA, ebpS, eno and cna) and biofilm-related protein Bap (bap) in Staphylococcus spp. isolates was studied using PCR (Eveline et al., 2015). The rate of recurrence of fib, fnbA, eno and bap were elevated in contrast to the other appraised genes (fnbB, ebpS and cna). The elevated rate of occurrence of the bap gene in CNS in contrast to CPS proposes that in these species biofilm development is a crucial mechanism for the tenacity of the infection. The medians of the somatic cell counts (SCCs) in the samples where fib, fnbA, eno and bap genes were identified were elevated contrasted to Staphylococcus aureus without the appraised genes and negative samples, which showed that the manifestation of these MSCRAMM may be associated to an elevated strength of the inflammatory progression (Eveline et al., 2015).
Infections of Methicillin-resistant Staphylococcus aureus are crucial, the progression of a potent vaccine can assist in the prevention of this infection (Wacker et al., 2014). In a mouse model recombinant PBP2a was studied (Setareh et al., 2017). Recombinant PBP2a (Freund’s adjuvant containing 20 mg of r-PBP2a) was prepared and inoculated subcutaneously into Balb/c mice. ELISA technique was used to appraise in serum the complete and specific isotype antibodies. Opsonophagocytic ability were examined in the serum. Intraperitoneal challenge with a sublethal dose of MRSA (5 108 CFU) was performed in investigational mice. After this, the amount of microorganisms from the kidneys of the mice were evaluated. Substantial upsurge in antibody with elevated levels of IgG2a, IgG2b and IgG1 isotypes was established in immunized mice compared with the control group (Setareh et al., 2017). The microbial burden in the kidneys from vaccinated mice was 1000 times less than control group (PBS) and opsonophagocytic ability of vaccinated mice sera substantially increased. The life span of vaccinated mice following microbial challenge was prolonged compared with mice in the control group. The outcomes indicated the ability of PBP2a as a candidate for vaccine to avert the MRSA infections (Setareh et al., 2017).
S. aureus resistance to all beta-lactam antimicrobials and methicillin is as a result of the performance of penicillin binding protein2a (PBP2a) that is situated in the cell wall of resistant strains (Senna et al., 2003; Foster, 2004; Roth and Machado, 2006; Abdullah et al., 2015; Setareh et al., 2017). PBP2a is observed both in community acquired MRSA (CA-MRSA) and hospital acquired MRSA (HA-MRSA), mecA is accountable for the generation of PBP2a (Foster, 2004; García-Álvarez et al., 2011; Setareh et al., 2017). PBPs are enzymes bound to the membrane which catalyze the transpeptidation and is essential for peptidoglycan chains bridging (Lowy, 2003; Foster, 2004; Setareh et al., 2017). Dissimilar to all PBPs, PBP2a has a little proclivity to all beta-lactam antimicrobials that permits S. aureus to persist in the elevated levels of the antimicrobials (Peng et al., 2002; Foster, 2004; Setareh et al., 2017). As a result of the capability of MRSA to accomplish supplementary antimicrobial resistance, the therapy of these infections remains a tenacious task (McVicker et al., 2014; Setareh et al., 2017).
A vaccine is ought to be premeditated regarding to its virulence features indicated in the various stages of infection, in order to prevent against numerous syndromes elicited by the microorganism (Broughan et al., 2011; Nasim et al., 2017). S. aureus retain diverse forms of virulence features; accordingly, determinations in developing a potent vaccine against S. aureus manifested predominantly as ineffective (Adhikari et al., 2012; Nasim et al., 2017). Planning for a potent vaccine against dissimilar strains of S. aureus, several antigens should be carefully chosen. Furthermore, to increase the host immune responses, the vaccine must be complemented with an apposite adjuvant (Adamczyk-Poplawska et al., 2011). Subunit vaccines are a type of vaccine categories that have numerous benefits comprising of a distinct constituent, diverse methods of transport, devoid of harmful microorganisms, and innocuous existence (Harro et al., 2010; Nasim et al., 2017). Prospective antigens candidate, which are useful as subunit vaccines should possess numerous essential physiognomies; they should be preserved amongst diverse microbial genotypes and or serotypes and also be restricted on to the cell surface, where they are reachable to antibodies to stimulate a suitable immune response (Adamczyk-Poplawska et al., 2011; Adhikari et al., 2012; Nasim et al., 2017).
Incidentally, three determinants of antigen namely, iron surface determinant B (IsdB), clumping factor A (ClfA) and Alpha-enolase (Eno1) were appraised by the existing bioinformatics contrivances for planning of an effective multi-epitope subunit vaccine for the stimulation of immune responses against Staphylococcus aureus infections. A cell wall-fastened and multifunctional protein called Eno1, which is contained in the cytoplasm and observed superficially on several prokaryotic and eukaryotic cells, is abundant in all the strains of S. aureus verified and has an extremely preserved arrangement. Attachment of Eno1 to laminin, the most copious extracellular medium constituent showed a significant role in the pathogenesis of this bacterium (Pancholi, 2001; Ghasemi et al., 2016; Nasim et al., 2017).
ClfA is an additional cell wall-fastened protein, which is observed superficially on S. aureus and intercedes its attachment to γ-chain of host fibrinogen (García-Lara and Foster, 2009). Erstwhile research has divulged that ClfA has an essential role in the stimulation of Staphylococcus aureus infections (Brouillette et al., 2002; Vernachio et al., 2003; Nasim et al., 2017). Therefore, this virulence feature, as a vaccine constituent, affords a prospective objective for the stimulation of a strong active and passive immune response to avert staphylococcal infections (Brouillette et al., 2002; Brouillette et al., 2004; Ganesh et al., 2008; Nasim et al., 2017).
IsdB is the last determinant antigen. This proteinaceous antigen is attached to the cell wall and is seen superficially on the cell (Zapotoczna et al., 2013; Nasim et al., 2017).The protein is preserved amongst different strains of S. aureus, and is observed only under restrictive iron circumstances (Nasim et al., 2017; Schaffer and Lee, 2008). It could perchance be the most important virulence feature of S. aureus (Nasim et al., 2017; Kuklin et al., 2006) via attaching to haemoglobin and getting hold of heme iron from host’s haemoglobin (Nasim et al., 2017; Zapotoczna et al., 2013).
Nanoparticle conveyance structures are extensively explored clinically with numerous element-based formulations and contrivances that were frequently been used in the clinical setting (Torchilin, 2014; Min et al., 2015; Anselmo and Mitragotri, 2016; Halwani et al., 2016). In recent times attention of researchers is shifted toward the advancement in nanoparticles (NPs) as conveyance designed for vaccination. The vaccine Antigen is either embedded in the interior or applied onto NP exteriorly. Implantation of the vaccine antigenic substance, NPs would be able to afford an efficient transporting medium for antigens such that their half-life would be protracted and provide effective immune response. Conjugation of antigens onto NPs can permit presentation of the immunogen to the immune systems as would a pathogen, thus inducing a similar response. ISCOMs, liposomes, VLPs, non-degradable NPs and polymeric NPs are paramount as conveyance structures for bacterial proteins.
Bioinformatics contrivances assist many canvassers in a broad spectrum of biological meadows (Nasim et al., 2017; Negahdaripour et al., 2016; Rahmatabadi et al., 2016); exclusively, for the selection of apposite vaccine candidates, immunoinformatics investigations are certainly valuable (Zagursky et al., 2003; Nasim et al., 2017). In a recent study, a new multi-epitope subunit vaccine, which is a mixture of numerous B-cell, T-cell, and IFN-γ stimulating epitopes, was made to stimulate a strong innate, humoral and cellular immune responses against the harmful S. aureus comprising the antimicrobial-resistant infections. Currently, molecular dynamics (MD) replication is comprehensively utilized to get an improved perspicacity of biological procedures (Hansson et al., 2002; Nasim et al., 2017). In the research of Nasim et al. (2017), MD replication procedure was used to observe the vaccine behavior and its steadiness concerning a receptor. The vaccine and receptor molecules were consistent and thus, institute the finest approach to interactions throughout the MD replication period (Nasim et al., 2017). Current developmental status of Staphylococcus aureus vaccine candidates were similarly documented (Table 1) (Birgitte et al., 2016).

Figure 2: Functions of vaccine particle preparations in regulating vaccine systemic delivery and presentation to immune cells. Vaccines particles in the size range of 20 to 100 nm are particularly transported into the lymphatic vessels towards the Lymph nodes (LNs), whereas smaller molecules are distributed into the systemic circulation with inferior lymphatic uptake. Bigger particles become entombed in tissue and incline to be deposited close to the site of injection. (1) Vaccines particles comprising of both antigen and the danger signals can be coded by the liver these vaccine components are presented to the APC, permitting the stimulation of PRRs selectively in cells that have received antigen. (2) Enormous population of antigen on the surface of vaccines particles augments cross-linking of antigen receptors on specific B cells. Source: Moyer et al., 2016 with some modifications.
Transcriptomics,Metabolomics, Metagenomics, and Proteomics as Promising Procedures For The Advancement of Vaccines Candidate as Anti- Staphylococcus Aureus in Ruminants Proteomics and Transcriptomics:
Due to the current advancement in transcriptomics and proteomics exploration, an extensive appraisal of the immeasurable proteins and genes that were revealed by microorganism is currently manageable. Transcriptomics and proteomics research remain crucial development in varying genetic facts activity of the cell and protein alteration. Wide-ranging complete set of genes pertaining to transcriptome research disclosed the manifestation of virtually 70/100 of microbiological copies of gene (Adamu et al., 2016; Nelson et al., 2008). The proteomics research concerning 1 and 2-D gel documented from Arthropods and mammals cells acquired from the cultures of microbes was about 1/4 of the intact open reading frames (Singu et al., 2005; Seo et al., 2008; Adamu et al., 2016). On the other hand, there were some prevailing complications in proteomic exploration with reference to bacteria; due to remarkable effort involved in locating extremely refined sample, and presence of huge amount of proteins in the host reduces the capability of exposure and the responsiveness eminence of microbiological proteins (Li and Lostumbo, 2010; Adamu et al., 2016). The development of tandem MS/MS (nano-LC–MS/MS)-based proteomic in addition to the extremely responsive nano-liquid chromatography method augments protein estimation of microbes, for instance small amounts of proteins are able to be identified in samples mixed with a substantial measure of proteins from the host (Zimmer et al., 2006; Adamu et al., 2016). Proteomic and transcriptomics methods may perhaps be effective in the advancement of vaccine candidates owing to their relative protein abundance. Appraisal of protein expression of microbes could assist in augmenting the comprehension of the pathophysiology of microbes, and the convoluted modification amongst the host and the microbes, and increase the probabilities for the assessment of new targets for a powerful immunogenic vaccine candidate as anti- Staphylococcus aureus.
Metabolomics and Metagenomics
Metabolomics and metagenomics procedures have improved and may perhaps appear propitious in identifying immunogenic antigen as a vaccine candidate of Staphylococcus aureus, transmitting ground-breaking vision for the development of potent vaccines. Investigation in metagenomics, and the review on microbes and their association in welfare and contagion may perhaps immeasurably remain intensified using this procedure. Metagenomics assessments are classically accomplish by sequencing the microbial 16S and 18S ribosomal RNA (rRNA) subunit or the intact metagenome shotgun sequencing, characteristically on an enormously analogous platform of pyrosequencing (Dave et al., 2012; Adamu et al., 2016; Gulani et al., 2016).
Table 1: Developmental status of current Staphylococcus aureus vaccine candidates
|
Candidate
name/Identifier |
Developer | Vaccine approach | Pre- clinical | Phase I | Phase II | Status |
| Active prophylactic vaccines | ||||||
| PF-06290510/SA4Ag | Phizer |
ClfA/MntC/CP5/CP8 conjugated to CRM197 |
X | Safety and Efficacy of SA4Ag Vaccine in Adults Having Elective Posterior Instrumented Lumbar Spinal Fusion Procedure (STRIVE): NCT01827358 | ||
|
X
|
SA4Ag Safety, Tolerability, and Immunogenicity Study in Japanese Adults: NCT02492958 | |||||
| GSK2392103A | GSK |
CP5/CP8/TT/AT/ClfA plus AS03B |
X
|
No longer under active development. A Study to Evaluate the Safety, Reactogenicity and Immunogenicity of GSK Biologicals’ Staphylococcal Investigational Vaccine in Healthy Adults: NCT01160172 (Singu et al., 2005). | ||
| NDV3 | NovaDigm Therapeutics |
rAls3p-N (C.albicans surface protein that cross reacts with S. aureus) plus Alum |
X
|
Under development. Safety and Immunogenicity Study of a Recombinant Protein Vaccine (NDV-3) Against S. aureus and Candida: NCT01273922. Clinical development for Vulvovaginal candidiasis (VVC) ongoing: NCT01926028 (So-Yon et al., 2006). |
||
|
Glycosylated CP5, CP8, and HlaH35L |
GSK (Glycovaxyn) | X | ||||
| SA75 |
Vaccine Research International |
Whole cell vaccine | X |
No longer under active development http:// www.vri.org.uk/PhaseITrial.pdf (Staats et al., 2001) |
||
| 4C-Staph | Novartis |
FhiD2, EsxAB, Hla, Sur-2 |
X | |||
| Pan Chai University |
S. aureus ghosts |
X |
||||
| various | IBT/NIAID |
Multi-valent attenuated toxoid |
X | (Negahdaripour et al., 2016) | ||
| Passive prophylactic immunization | ||||||
| MEDI4893 | Medimmune |
mAb binding to S. aureus toxin |
X
|
Dose-ranging efficacy and safety in mechanically Ventilated Adults: NCT02296320 | ||
| AR-301 | Aridis | mAb |
X
|
Phase I/II Safety, Pharmacokinetics and Efficacy of KBSA301 in Severe Pneumonia (S. aureus) as an adjunctive therapy to standard of care antibiotics in hospital-acquired pneumonia (HAP) and ventilator associated pneumonia (VAP) patients: NCT01589185 |
||
(Birgitte et al., 2016)
The data has extended the possibility of culture-dependent microbiological procedures and has boosted the understanding of microbial units that got embedded in the organs, tissues and the systemic circulation and the reasons of microbes interdepending on the host (Dave et al., 2012; Adamu et al., 2016; Gulani et al., 2016).
Wide-ranging metagenome or metatranscriptome shotgun sequencing (WMS) is the composition of all nucleotides collated, catalogued microbes that may possibly emerge as a species or strain whereas conveying pro-active established indication on genomic content. The downsides of WMS contains inflated financial proportion of the nucleotide amount and funds required to estimate the enormous figures and the infection of host nucleic acid. WMS techniques retain an intrinsic nucleic acid exclusion presumption for instance an unspecified number of microbes are eliminated glibly comparative to some microbes. It is additionally operative than Sanger sequencing, of the “next-generation” abilities that maintain smaller reads and for that reason persist to be liable to sequencing inaccuracies (Morgan and Huttenhower, 2014; Adamu et al., 2016).
Eccentrically, metabolomics progressed as articulate and an objective appraised research of small molecular mass molecules, or metabolites, generated systemically in response to an organic stimulation. Metabolites are extravasated into systemic fluids by microbial and host cells, evaluated by spectrometry methods, and arrange alongside with collections of accredited biochemical. These procedures have been involved to realize the process of pathogenesis and the identification of new marker of disease. Metabolomics consistently stipulates the manifestation and the purpose of microbes existing in challenging crannies and high spot diverse connection amongst host, microbial metabolism, and comparative welfare or contagion (García-Álvarez et al., 2011; Adamu et al., 2016).
Metabolites generated by host and microbial cells consist of unusual assortment of physical and chemical physiognomies, and may be obtainable systemically and are acquire in disparate measures. Successively, no precise platform of metabolomics is capable to classify all metabolites in a specimen, accordingly dissimilar technique are recurrently exploited (Dettmer et al., 2007; Yozwiak et al., 2012; Adamu et al., 2016). Mass spectrometry linked to gas chromatography (GC-MS) identifies unstable, thermally constant metabolites with responsiveness of less than millimolar, while liquid chromatography (LC-MS) is exploited to categorize non-volatile polar and non-polar compounds by means of nanomolar ability. The techniques remain contingent on specimen formulation procedures that indicate preconception and unavoidably metabolite are misplaced (Geoffrey and Peter, 2015; Adamu et al., 2016). Nuclear magnetic resonance (NMR) spectroscopy doesn’t need initial cataloguing of features in a specimen, nonetheless restraining specimen formulation decreases the resolution; NMR can typically identify features at or above a millimolar level. The main downsides to all metabolomics methods is budget reliant, both in terms of material procurement and volume of labor complicated in the data assessment. Additionally, an abundantly interpreted and an all-inclusive metabolite library, explicitly for microbial derived compounds, is still restricted (Geoffrey and Peter, 2015; Adamu et al., 2016).
ACKNOWLEDGEMENTS
Certainly, hereby we acknowledged the efforts of Encik Jefri M.N, Encik Mohd-Fahmi Mashuri and Ashe Abdullahi Abdulrahaman during the process of writing this manuscript.
CONFLICT OF INTEREST
This manuscript has not been submitted for publication elsewhere and been approved by all co- authors. The authors declare that they do not have any conflict of interest.
Authors COntribution
All the authors have equal contribution in the development of the manuscript.
REFERENCES





