Advances in Animal and Veterinary Sciences
Research Article
Detoxification of Aflatoxin B1 by Ginger Extract in Mice
Dalia Abdul-Kareem Abdul-Shaheed2, Oday Sattar Abbas1*
1University of Baghdad /College of Veterinary Medicine, Baghdad, Iraq; 2Ibn Sina University of Medical and Pharmaceutical Sciences, Baghdad, Iraq.
Abstract | Aflatoxin B1 (AFB1), is a mycotoxin found in food and feed, exerts harmful effects on humans and animals. The liver is the earliest target organ of AFB1, and its effects have been evaluated in animal models exposed to acute or chronic doses. For this reason, this study was planned to detoxify the AFB1 by ginger root extracts in mice. Forty mice were caged to four experimental groups and treated for 5 weeks with extracts and with or without AFB1. Blood samples were collected and removal the livers at the end of an experimental period for serum biochemical analysis of Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), and Gamma-glutamyl transpeptidase (GGT) and study the histopathological changes of the liver. The results indicated that mice treated with AFB1 alone showed a significant increase (P≤0.05) in all parameters tested (79.01± 1.05 IU/L), (199± 1.15 IU/L), and (27.19±0.97 IU/L) respectively, whereas no significant differences were noticed in group treated extract alone while in group treated ginger root extract with AFB1 could significantly lower the serum activity level of all parameters tested to reach (38.10± 1.01 IU/L), (94.96± 1.22 IU/L), and (15.08±1.21 IU/L) respectively. The histopathological changes of liver showed congestion of central vein, apoptotic cells and formation of a mitotic figure in a group treated AFB1 alone, whereas group treated AFB1 with extract showed regeneration in most hepatocytes normal structure. These results clearly indicated that AFB1 has stressful effects on the hepatic tissues and ginger root extract was found most effective in the prevention of Aflatoxin-induced toxicity in mice.
Keywords | Aflatoxin B1, Ginger root extracts, Aspergillus mycotoxin, Liver toxicity, Detoxification of mycotoxin.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 28, 2018; Accepted | August 12, 2018; Published | September 01, 2018
*Correspondence | Oday Sattar Abbas, University of Baghdad /College of Veterinary Medicine, Baghdad, Iraq; Email: droddr7757@gmail.com
Citation | Abdul-Shaheed DAK, Abbas OS, (2018). Detoxification of aflatoxin b1 by ginger extract in mice. Adv. Anim. Vet. Sci. 6(9): 384-387.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.9.384.387
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Abdul-Shaheed and Abbas. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Aflatoxin is secondary metabolites of certain strains of Aspergillus flavus and Aspergillus parasiticus that have been shown to be toxigenic, carcinogenic, mutagenic, and teratogenic to different species of animals (Hoffmann et al., 2015). Aflatoxin has been detected in cereal grains, whole wheat and rye bread, oilseeds, fermented beverages made from grains, milk, cheese, meat, nut products, fruit juices, and numerous other agricultural commodities (John, 2015). Therefore, the presence of Aflatoxin or toxigenic fungi in foods presents a potential hazard to human and animal health. Human epidemiology and experimental animal studies have provided the statistical association and biological information necessary to suggest that Aflatoxin are risk factors for human liver cancer (IARC, 2012), its activated by cytochrome P450 to form Aflatoxin B1-8,9- epoxide, which is responsible for the mutagenic activity, Aflatoxin B1-8,9 epoxide specifically binds to the N7 position of guanine of DNA and RNA to form Aflatoxin B1-N7-guanine adduct (Voth-Gaeddert et al., 2018). Aflatoxin B1 inhibits DNA, RNA, and protein synthesis, resulting in immuno-suppressive, hormonal and teratogenic effects (Aiko and Mehta, 2015).
Ginger (Zingiber officinale) is a flowering plant whose rhizome, ginger root or simply ginger, is widely used as a spice or a folk medicine, it’s a herbaceous perennial which grows annual pseudostems (false stems made of the rolled bases of leaves) about a meter tall bearing narrow leaf blades. Ginger is in the family Zingiberaceae, to which also belong turmeric (Curcuma longa), cardamom (Elettaria cardamomum), and galangal (An et al., 2016). Ginger is renowned for its multiple medicinal uses. Ancient Hawaiian medicine recommends ginger to relieve a headache, toothache, achy joints, and stomachache as well as various other maladies (Wen et al., 2014). Modern herbal medicine uses ginger primarily to treat motion sickness, however, its anti-inflammatory properties can also help to treat migraine headaches, rheumatic and muscular disorders, arthritis, and other inflammation-related ailments and it’s a potentially very effective phytomedicine in treating a wide variety of illnesses (Chinese Pharmacopoeia, 2010).
MATERIAL AND METHODS
Chemicals
All chemicals were purchased from Sigma company and the standard Aflatoxin B1 (AFB1) purchased from Santa Crusz Company (USA).
Animals and Experimental Design
A total forty albino BALB/C male mice average weight (30±5g) were used. Animals were caged randomly allocated into four experimental groups and given water and fed wheat. The first group of 10 mice was (Control) were fed on uncontaminated wheat; the second group of 10 mice served as the treated group were fed on contaminated wheat with 35μg/ kg body weight AFB1 for 5 weeks served as positive control, the third group of 10 mice was fed on ginger root extract alone (30ml/kg body weight) and the fourth group of 10 mice was fed on a mix of ginger root extract and contaminated wheat with 35μg/ kg body weight AFB1 for 5 weeks. At the end of the experimental period, the mice were sacrificed. Blood samples were collected in sterile plastic test EDTA tube and serum samples were separated and used for analysis of biochemical parameters.
Extraction of Ginger Root Extract (GRE):
Ginger root (Zingiber officinale) were obtained from Baghdad markets and the extraction was performed (Angeli et al., 2014). Ginger samples were ground using an electric blender, weighed, and mixed with a solvent, and extracted by 70% ethanol in conical flasks sealed with foil and put on a magnetic stirrer for 7 days. Then, they were filtered to obtained crude extracts. The extract was then placed into open Petri dishes in an incubator at 40˚C until dryness. All extract was stored at -18˚C when they not use.
Serum Analysis
All biochemical parameters for liver function: Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST) and Gamma-glutamyl transpeptidase (GGT) were determined with the blood autoanalyzer Mindray BC-2800 (Germany) using kits (DPC, Diagnostic Products Corporation, USA) in Al- Yarmouk hospital.
Histological Examination
Liver processing and staining depending on (Luna, 1968).
Statistical Analysis
The data were analyzed using one way ANOVA. Differences were considered significant at P < 0.05.
RESULTS
Biochemical Results
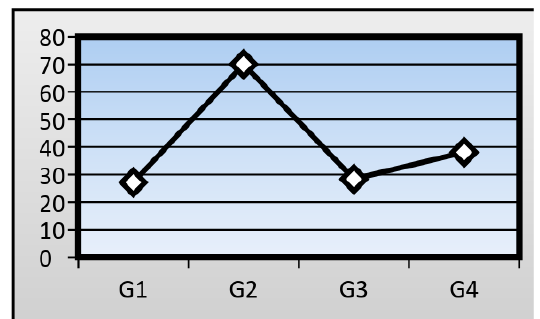
Serum levels of alanine aminotransferase activity (ALT): The mean serum activity level of (ALT) in treated group on which the mice fed on AFB1 contaminated wheat (79.01± 1.05 IU/L), where significantly higher (P≤ 0.05) than control mice (27.15 ± 1.12 IU/L). When ginger root extract used with AFB1, the ginger root extract could significantly lower the serum activity level of ALT to reach (38.10± 1.01 IU/L), but significantly higher than that of control group, while the serum activity level of ALT in mice treated with ginger root extract alone reach to (28.34± 1.00 IU/L) (Figure 1).
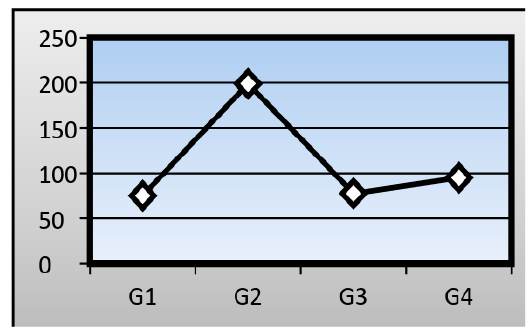
Serum levels of Aspartate Aminotransferase (AST): There was a significant change in the mean serum level of (AST) in the AFB1 group (199± 1.15 IU/L) when compared to that of a control group (75.03± 1.05 IU/L). The ginger root extract with AFB1 group has been recorded also a significant difference in the mean serum level of (AST) (94.96± 1.22 IU/L) when compared to that of a control group and AFB1 group, while the ginger root extract recorded about (77.61± 0.98 IU/L) (Figure 2).
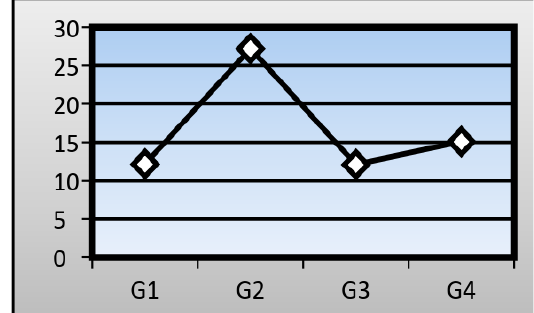
Serum levels of Gamma-glutamyl transpeptidase (GGT): There was a significant difference in the mean serum level of (GGT) in the AFB1group (27.19±0.97 IU/L) in comparison with that of a control group (12.14±1.01 IU/L). Ginger root extract plus AFB1group showed no significant difference in the mean serum level of (GGT) (15.08±1.21 IU/L) into that of a control group, but mean serum level of (GGT) in mice treated with ginger root extract alone reached to (12.09± 1.03 IU/L) (Figure 3).
:
Histopathological Result of Liver
Investigation of liver microscopic sections for a control group showed normal tissue (Figure 4). While mice treated with AFB1 contaminated wheat showed injury in the liver due to toxicity by showing enlargement of hepatocytes and infiltration of mononuclear cells in and around congested blood vessels with apoptotic and mitotic figures of hepatocyte (Figure 5). The mice fed on ginger root extract with AFB1showed less to mild signs (Figure 6).
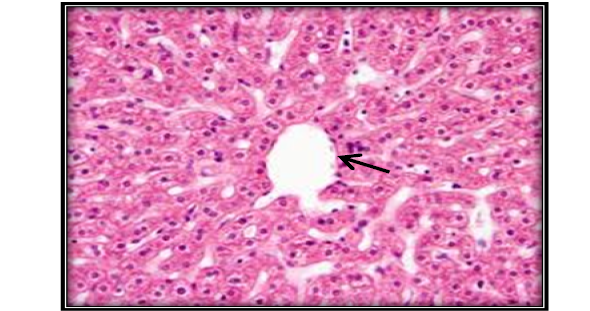
Figure 4: Liver section for untreated mouse (control) showing central vein () surrounded with hepatic cord (H&E 200X)
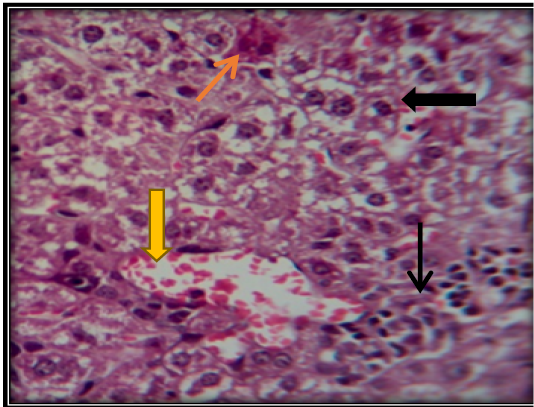
Figure 5: Liver section for mouse treated with AFB1showing congestion of central vein ( ) with vacuolation of hepatocyte ( ) and apoptotic cells ( ) and accumulation of inflammatory cells in necrotic areas ( ) (H&E 200X).
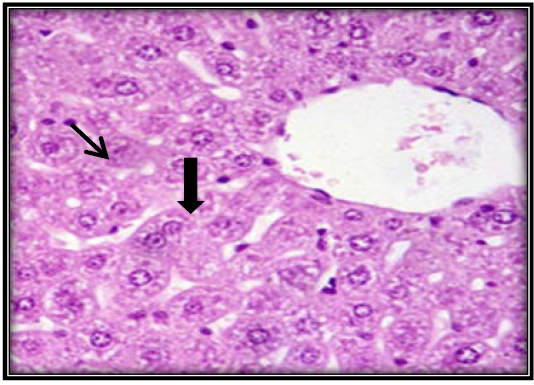
Figure 6: Liver section for mouse treated with AFB1 and ginger root extract showing single necrotic hepatocyte ( ) and mitotic figures of hepatocyte ( ) (H&E 400X).
DISCUSSION
The liver is considered to be the principal target organ for Aflatoxin. The activity of ALT and AST are sensitive indicators of acute hepatic necrosis. In the present study demonstrated that the treatment with ginger root extract effectively protected the mice against AFB1 induced hepatotoxicity, as evidenced by decreased AST, ALT and GGT levels. These protective effects of ginger root extract against AFB1 induced hepatotoxicity were also confirmed by histopathological investigation. Increased in transaminases (ALT, AST, and GGT) were considered as an indicative for changes in the hepatic tissues (Abdel-Wahhab and Aly, 2005), reflected hepatocellular damages and as a sensitive indicator of chronic hepatic necrosis, also these results clearly indicated that AFB1 has stressful effects on the hepatic tissues (Shaji and Harish, 2014).
Ginger is a potentially very effective phytomedicine in treating a wide variety of illnesses and it’s not only an important spice but also a traditional Chinese medicine. Firstly, ginger has been used since antiquity as food additives for the purpose of flavoring. Mehrim et al. (2006) indicated that ginger was the best detoxifying agent of Aflatoxin using 0.5% ginger as dietary additives to alleviate the toxic effects of AFB1 contaminated fish diets.
ACKNOWLEDGMENTS
We appreciate the laboratories of the Medical City and Al- Yarmouk hospital for its valuable cooperation in this research.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHORS CONTRIBUTION
Dalia Abdul-Kareem Abdul-Shaheed: Plan of work, serum biochemical parameters analysis, statistical analysis, and manuscript preparation.
Oday Sattar Abbas: Plan of work, execution, histopathological technique, and manuscript preparation.
REFERENCES